

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A3 receptor as inhibition of [125I]-AB-MECA binding to human receptor expressed in HEK 293 cells | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50070866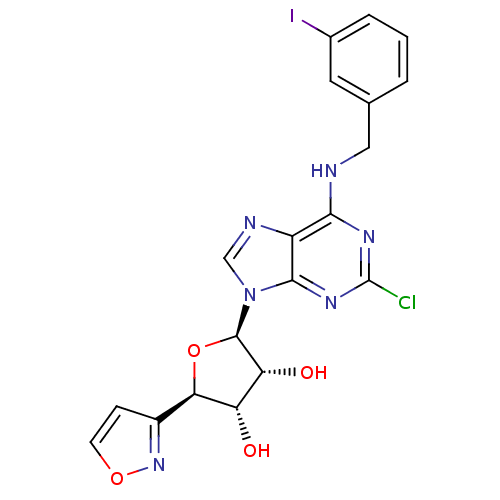 ((2R,3R,4S,5R)-2-[2-Chloro-6-(3-iodo-benzylamino)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A3 receptor as inhibition of [125I]-AB-MECA binding to human receptor expressed in HEK 293 cells | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50070867 ((2R,3R,4S,5R)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A3 receptor as inhibition of [125I]-AB-MECA binding to human receptor expressed in HEK 293 cells | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50070864 ((2R,3R,4S,5S)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A3 receptor as inhibition of [125I]-AB-MECA binding to human receptor expressed in HEK 293 cells | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50070865 ((2R,3R,4S,5R)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A3 receptor as inhibition of [125I]-AB-MECA binding to human receptor expressed in HEK 293 cells | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50070863 ((2S,3S,4R,5R)-5-(6-Benzylamino-purin-9-yl)-3,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A3 receptor as inhibition of [125I]-AB-MECA binding to human receptor expressed in HEK 293 cells | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A1 receptor as inhibition of 3[H]-R-PIA binding in rat brain | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM21221 ((2S,3S,4R,5R)-5-(2-chloro-6-{[(3-iodophenyl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A2a receptor as inhibition of 3[H]-CGS 21680 binding in rat striatum | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50070864 ((2R,3R,4S,5S)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A1 receptor as inhibition of 3[H]-R-PIA binding in rat brain | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50070865 ((2R,3R,4S,5R)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A1 receptor as inhibition of 3[H]-R-PIA binding in rat brain | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50070867 ((2R,3R,4S,5R)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A1 receptor as inhibition of 3[H]-R-PIA binding in rat brain | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50070866 ((2R,3R,4S,5R)-2-[2-Chloro-6-(3-iodo-benzylamino)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A2a receptor as inhibition of 3[H]-CGS 21680 binding in rat striatum | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50070866 ((2R,3R,4S,5R)-2-[2-Chloro-6-(3-iodo-benzylamino)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A1 receptor as inhibition of 3[H]-R-PIA binding in rat brain | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50070863 ((2S,3S,4R,5R)-5-(6-Benzylamino-purin-9-yl)-3,4-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A2a receptor as inhibition of 3[H]-CGS 21680 binding in rat striatum | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50070863 ((2S,3S,4R,5R)-5-(6-Benzylamino-purin-9-yl)-3,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A1 receptor as inhibition of 3[H]-R-PIA binding in rat brain | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50070864 ((2R,3R,4S,5S)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A2a receptor as inhibition of 3[H]-CGS 21680 binding in rat striatum | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50070865 ((2R,3R,4S,5R)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A2a receptor as inhibition of 3[H]-CGS 21680 binding in rat striatum | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50070867 ((2R,3R,4S,5R)-2-(2-Chloro-6-methoxyamino-purin-9-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Binding affinity for adenosine A2a receptor as inhibition of 3[H]-CGS 21680 binding in rat striatum | Bioorg Med Chem Lett 8: 1767-70 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130727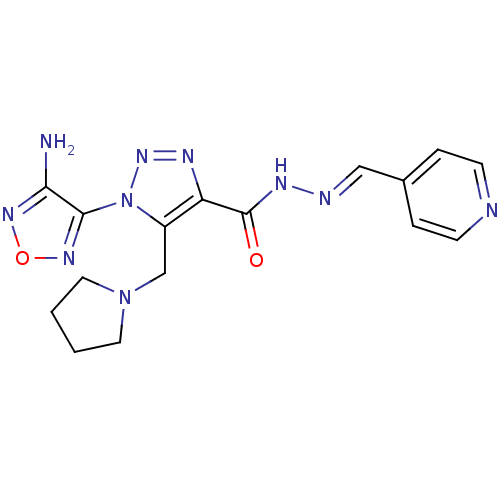 (1-(4-Amino-furazan-3-yl)-5-pyrrolidin-1-ylmethyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130724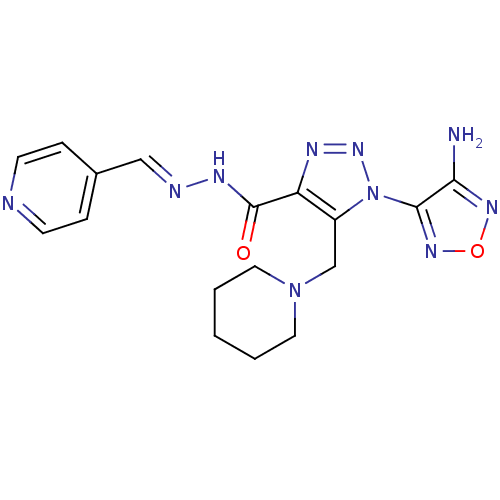 (1-(4-Amino-furazan-3-yl)-5-piperidin-1-ylmethyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130732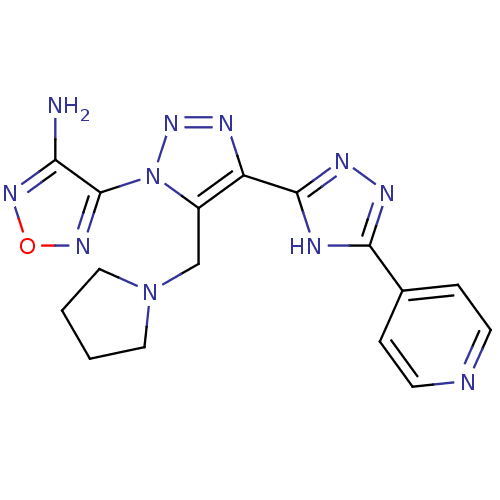 (4-[4-(5-Pyridin-4-yl-4H-[1,2,4]triazol-3-yl)-5-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130719 (4-[5-Piperidin-1-ylmethyl-4-(5-pyridin-4-yl-4H-[1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50130725 (3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2-cyclin A | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130725 (3-(3-Chloro-4-hydroxy-phenylamino)-4-(2-nitro-phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130730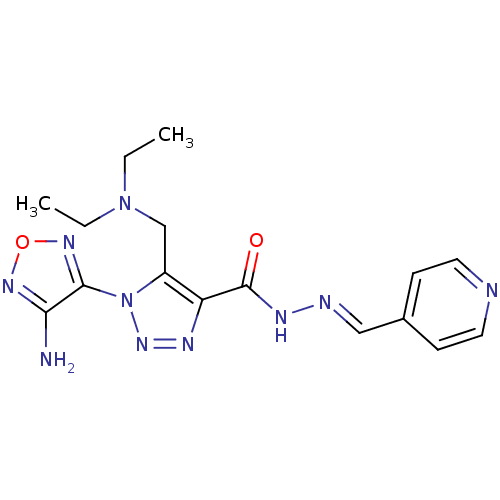 (1-(4-Amino-furazan-3-yl)-5-diethylaminomethyl-1H-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130720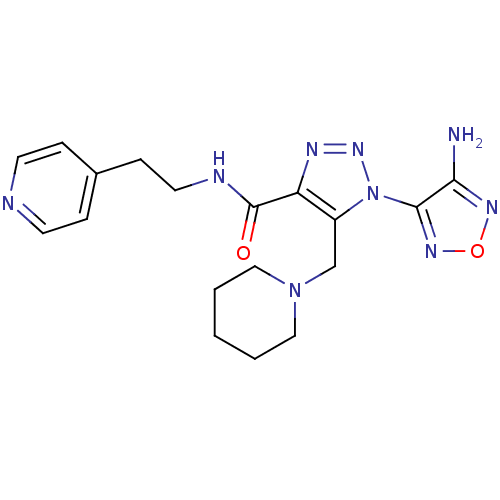 (1-(4-Amino-furazan-3-yl)-5-piperidin-1-ylmethyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130726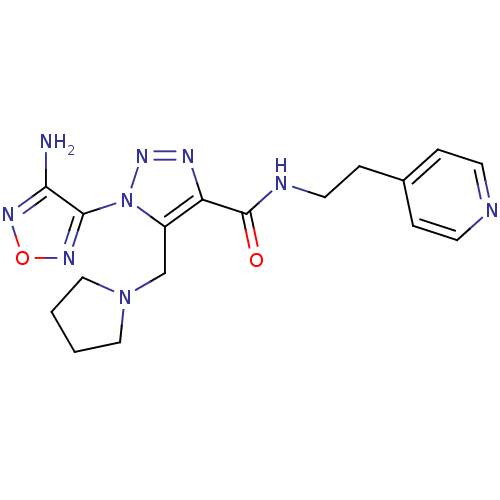 (1-(4-Amino-furazan-3-yl)-5-pyrrolidin-1-ylmethyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130722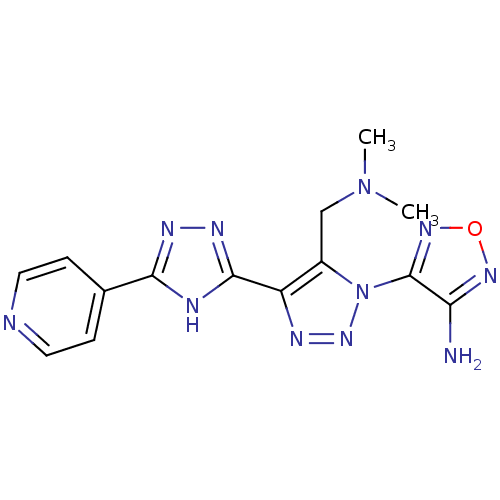 (4-[5-Dimethylaminomethyl-4-(5-pyridin-4-yl-4H-[1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130733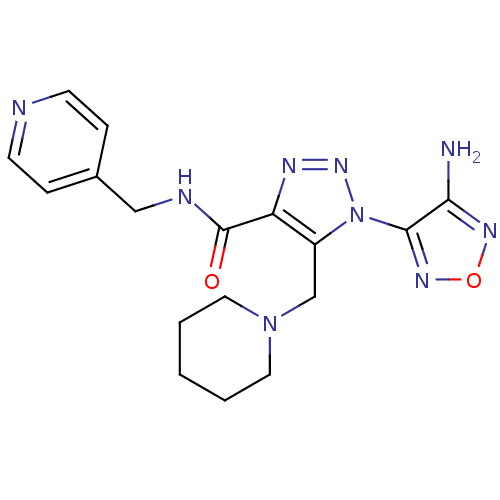 (1-(4-Amino-furazan-3-yl)-5-piperidin-1-ylmethyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130731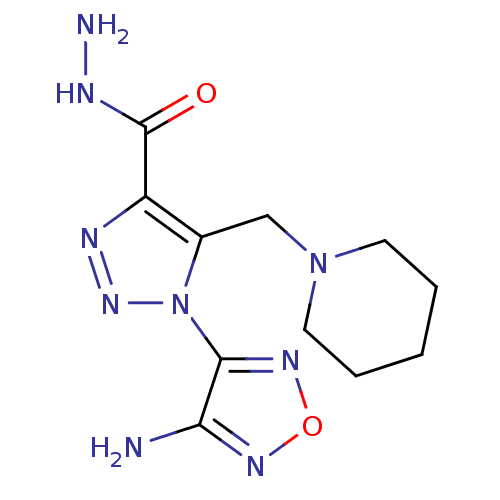 (1-(4-Amino-furazan-3-yl)-5-piperidin-1-ylmethyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130728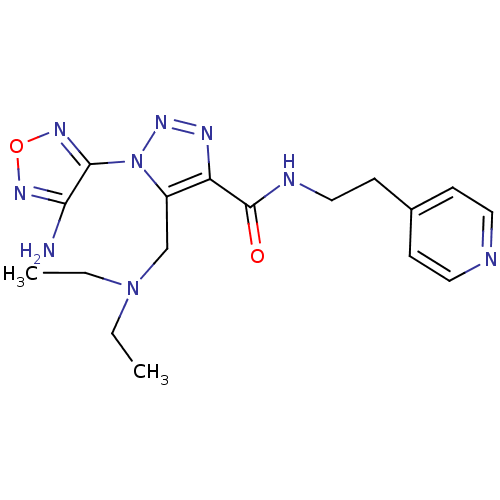 (1-(4-Amino-furazan-3-yl)-5-diethylaminomethyl-1H-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130721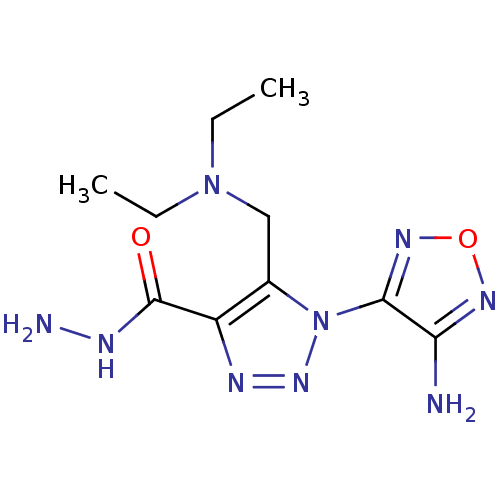 (1-(4-Amino-furazan-3-yl)-5-diethylaminomethyl-1H-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of human Glycogen synthase kinase-3 beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50130724 (1-(4-Amino-furazan-3-yl)-5-piperidin-1-ylmethyl-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2-cyclin A | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50130720 (1-(4-Amino-furazan-3-yl)-5-piperidin-1-ylmethyl-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2-cyclin A | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130729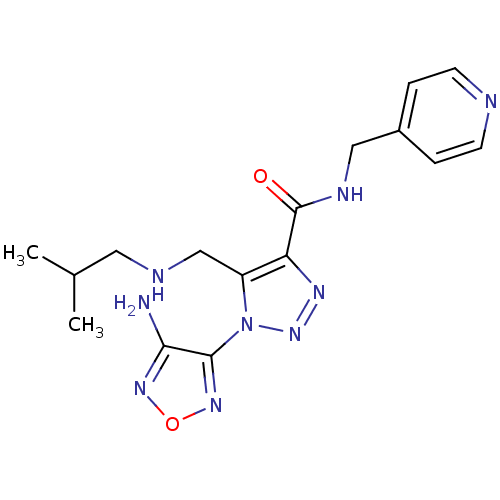 (1-(4-Amino-furazan-3-yl)-5-(isobutylamino-methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50130719 (4-[5-Piperidin-1-ylmethyl-4-(5-pyridin-4-yl-4H-[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2-cyclin A | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50130723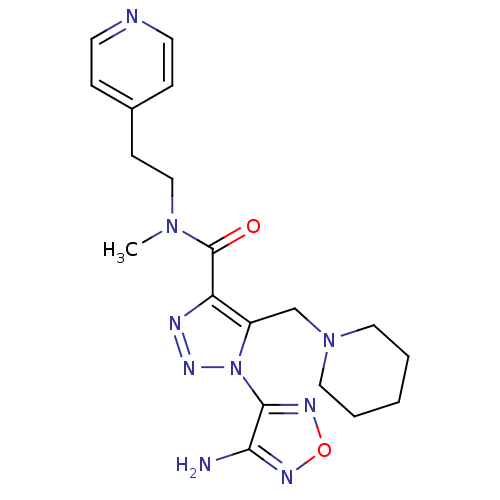 (1-(4-Amino-furazan-3-yl)-5-piperidin-1-ylmethyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against human glycogen synthase kinase-3beta (GSK3-beta) at 100 uM ATP | J Med Chem 46: 3333-41 (2003) Article DOI: 10.1021/jm021095d BindingDB Entry DOI: 10.7270/Q2F76D9B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||