

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50262661 (CHEMBL4075825) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262660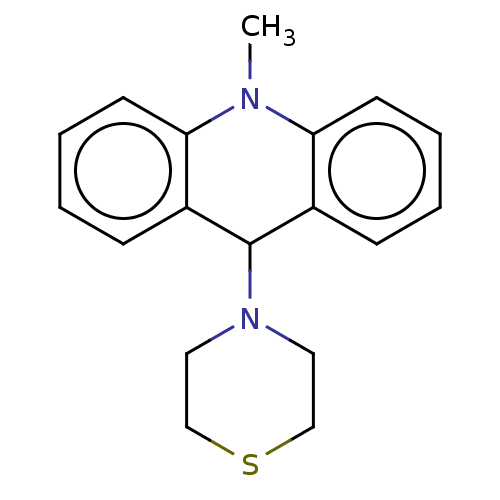 (CHEMBL4065259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262687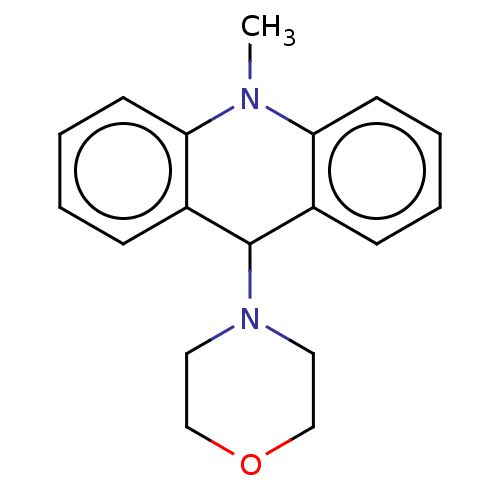 (CHEMBL4077169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262637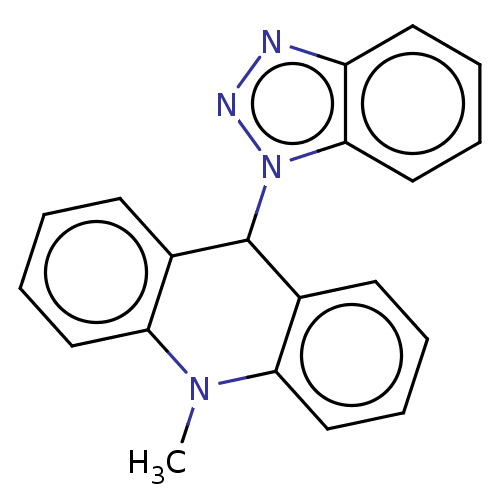 (CHEMBL4088659) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262651 (CHEMBL4080726) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262656 (CHEMBL4067342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262666 (CHEMBL4104952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262661 (CHEMBL4075825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262660 (CHEMBL4065259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262687 (CHEMBL4077169) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262637 (CHEMBL4088659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262651 (CHEMBL4080726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by Lineweaver-Burk double re... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262666 (CHEMBL4104952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262687 (CHEMBL4077169) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262661 (CHEMBL4075825) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262660 (CHEMBL4065259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262637 (CHEMBL4088659) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262662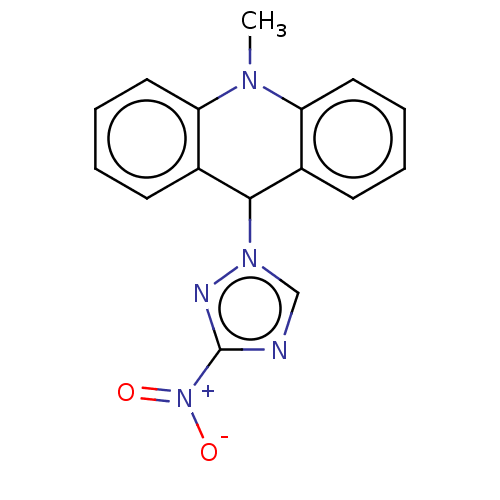 (CHEMBL4075624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50130915 (CHEBI:3122 | CHEMBL1231178) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262656 (CHEMBL4067342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's meth... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262651 (CHEMBL4080726) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262640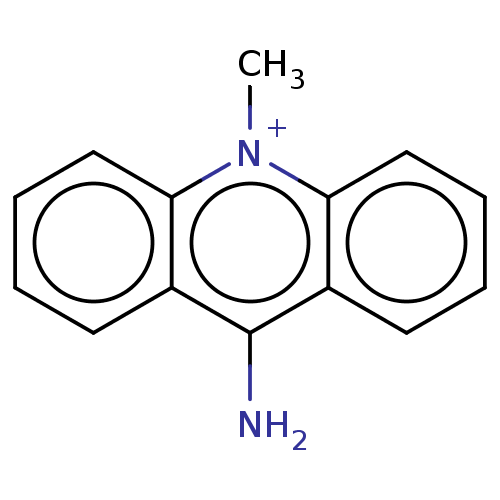 (CHEMBL4063635) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262666 (CHEMBL4104952) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262660 (CHEMBL4065259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262687 (CHEMBL4077169) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262637 (CHEMBL4088659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262638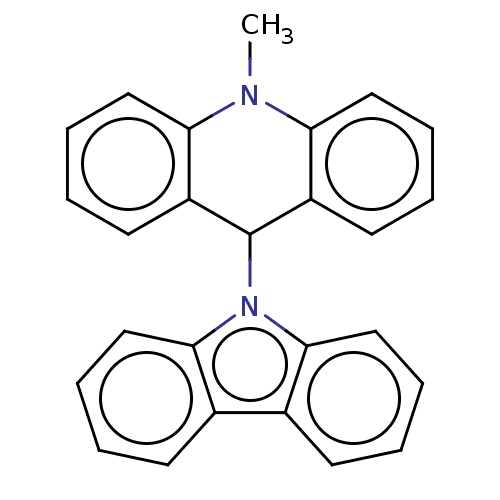 (CHEMBL4074993) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262655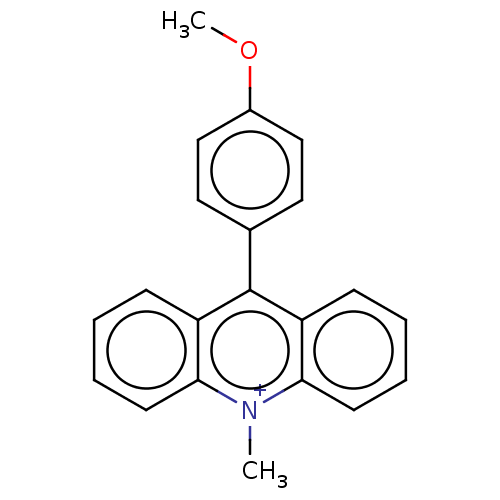 (CHEMBL4096311) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262636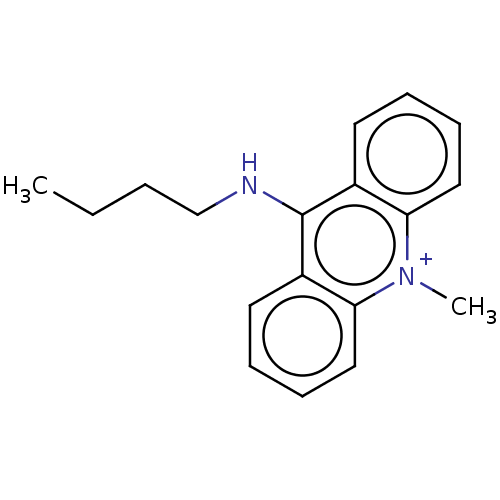 (CHEMBL4083288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262662 (CHEMBL4075624) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262661 (CHEMBL4075825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262652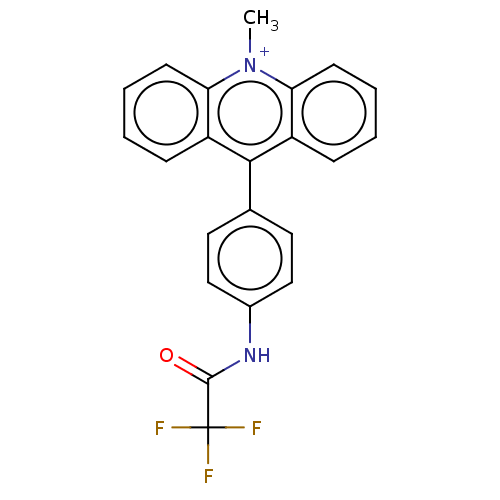 (CHEMBL4097249) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262648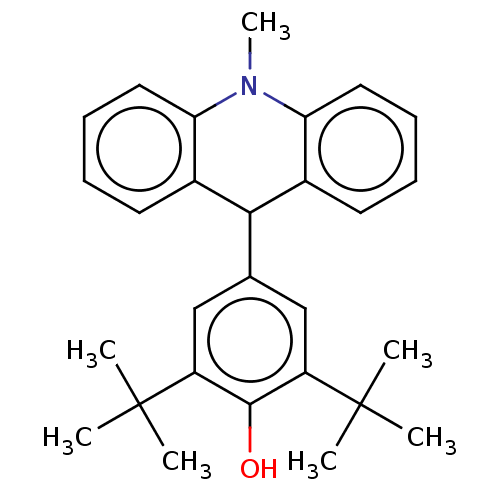 (CHEMBL4102576) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262647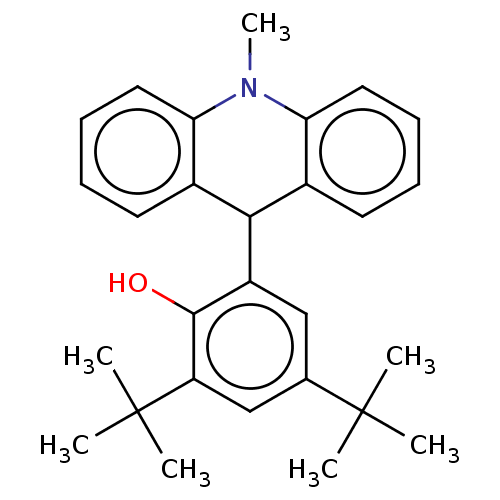 (CHEMBL4077493) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262646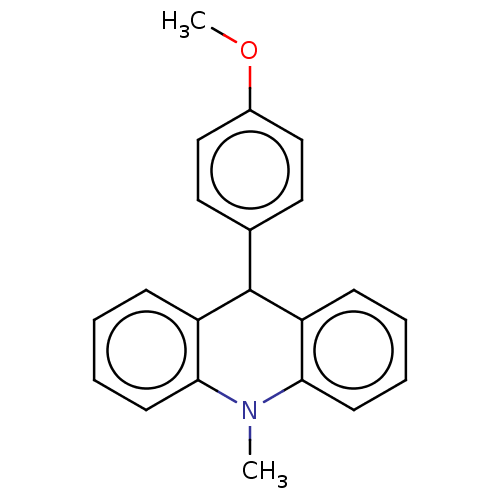 (CHEMBL4094837) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262645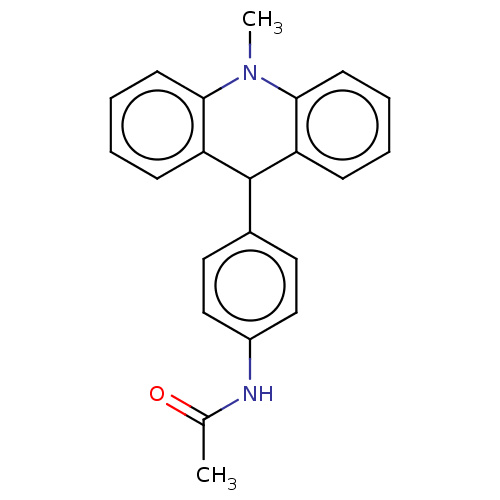 (CHEMBL4087149) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262644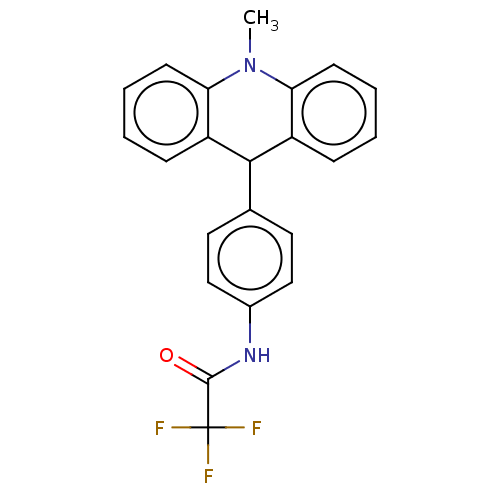 (CHEMBL4103528) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262642 (CHEMBL4080800) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262638 (CHEMBL4074993) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262636 (CHEMBL4083288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50262656 (CHEMBL4067342) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50262660 (CHEMBL4065259) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50262658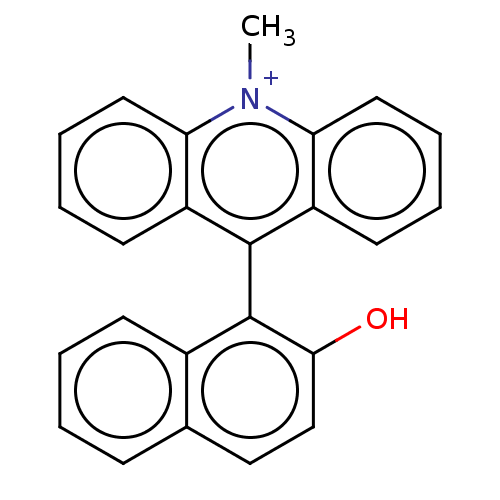 (CHEMBL4091935) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50262652 (CHEMBL4097249) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase (Sus scrofa) | BDBM50262691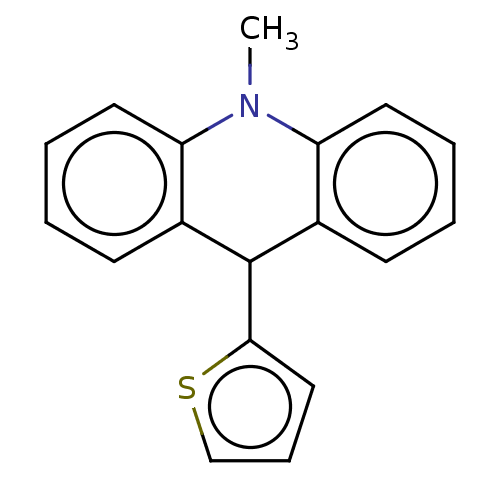 (CHEMBL4084629) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Inhibition of pig liver carboxylesterase using 4-nitrophenol acetate as substrate by spectrophotometric analysis | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262688 (CHEMBL4071110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262686 (CHEMBL4078265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50262691 (CHEMBL4084629) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Physiologically Active Compounds Russian Academy of Sciences, Chernogolovka 142432, Russia. Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by L... | Bioorg Med Chem 25: 5981-5994 (2017) Article DOI: 10.1016/j.bmc.2017.09.028 BindingDB Entry DOI: 10.7270/Q2FT8PHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 87 total ) | Next | Last >> |