 Found 359 hits with Last Name = 'bai' and Initial = 'b'
Found 359 hits with Last Name = 'bai' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM419133
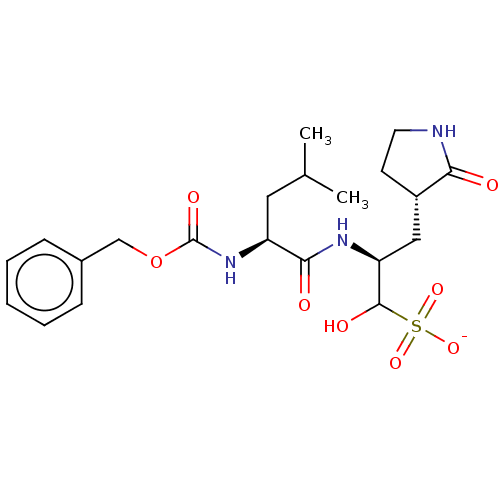
(BDBM429386 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50235359
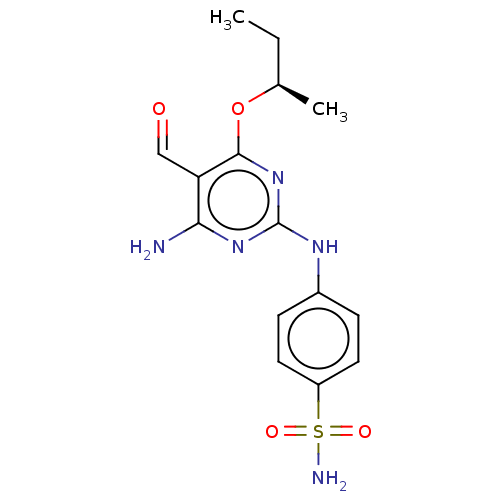
(CHEMBL4088242)Show SMILES CC[C@@H](C)Oc1nc(Nc2ccc(cc2)S(N)(=O)=O)nc(N)c1C=O |r| Show InChI InChI=1S/C15H19N5O4S/c1-3-9(2)24-14-12(8-21)13(16)19-15(20-14)18-10-4-6-11(7-5-10)25(17,22)23/h4-9H,3H2,1-2H3,(H2,17,22,23)(H3,16,18,19,20)/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476968

(SARS-CoV-2 3CLP and CoV inhibitor 15h)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)c1c(cccc1C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C34H36F6N4O7/c1-17(2)13-24(44-31(48)25-15-19-22(42-25)10-5-11-27(19)50-3)30(47)43-23(14-18-7-6-12-41-29(18)46)26(45)16-51-32(49)28-20(33(35,36)37)8-4-9-21(28)34(38,39)40/h4-5,8-11,15,17-18,23-24,42H,6-7,12-14,16H2,1-3H3,(H,41,46)(H,43,47)(H,44,48)/t18-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235343
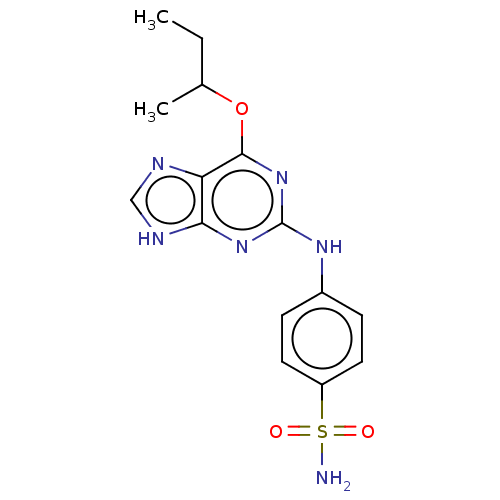
(CHEMBL4103249)Show SMILES CCC(C)Oc1nc(Nc2ccc(cc2)S(N)(=O)=O)nc2[nH]cnc12 Show InChI InChI=1S/C15H18N6O3S/c1-3-9(2)24-14-12-13(18-8-17-12)20-15(21-14)19-10-4-6-11(7-5-10)25(16,22)23/h4-9H,3H2,1-2H3,(H2,16,22,23)(H2,17,18,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged CDK2/bovine His-tagged Cyclin A |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235335
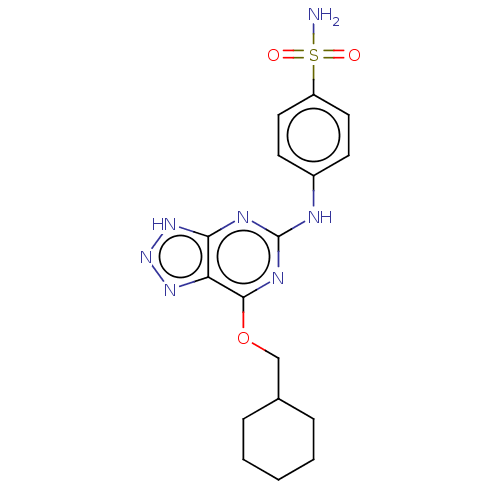
(CHEMBL4092981)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(OCC3CCCCC3)c3nn[nH]c3n2)cc1 Show InChI InChI=1S/C17H21N7O3S/c18-28(25,26)13-8-6-12(7-9-13)19-17-20-15-14(22-24-23-15)16(21-17)27-10-11-4-2-1-3-5-11/h6-9,11H,1-5,10H2,(H2,18,25,26)(H2,19,20,21,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50602863
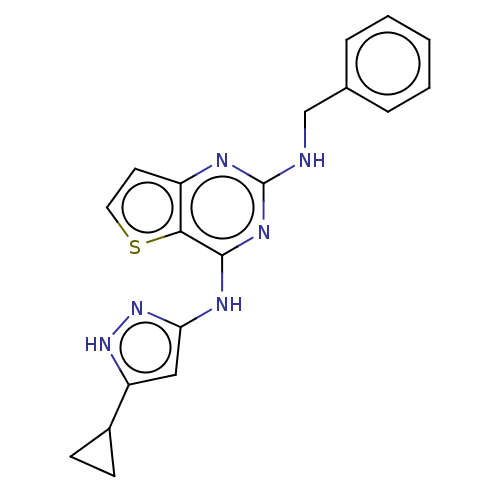
(CHEMBL1997924)Show SMILES C(Nc1nc(Nc2cc([nH]n2)C2CC2)c2sccc2n1)c1ccccc1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114054
BindingDB Entry DOI: 10.7270/Q20K2DM8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114054
BindingDB Entry DOI: 10.7270/Q20K2DM8 |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235348
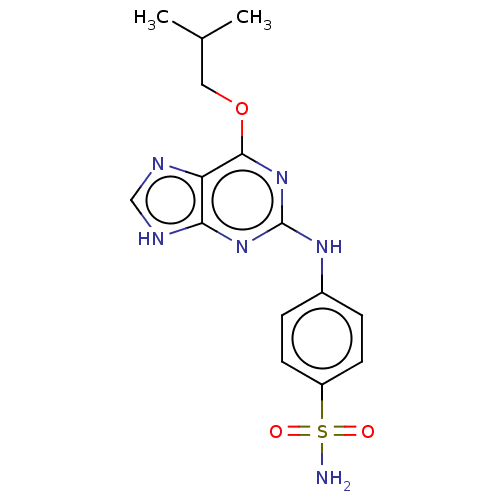
(CHEMBL4078988)Show SMILES CC(C)COc1nc(Nc2ccc(cc2)S(N)(=O)=O)nc2[nH]cnc12 Show InChI InChI=1S/C15H18N6O3S/c1-9(2)7-24-14-12-13(18-8-17-12)20-15(21-14)19-10-3-5-11(6-4-10)25(16,22)23/h3-6,8-9H,7H2,1-2H3,(H2,16,22,23)(H2,17,18,19,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged CDK2/bovine His-tagged Cyclin A |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476970

(SARS-CoV-2 3CLP and CoV inhibitor 15j)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)c1cccnc1 Show InChI InChI=1S/C31H37N5O7/c1-18(2)13-24(36-30(40)25-15-21-22(34-25)9-4-10-27(21)42-3)29(39)35-23(14-19-7-6-12-33-28(19)38)26(37)17-43-31(41)20-8-5-11-32-16-20/h4-5,8-11,15-16,18-19,23-24,34H,6-7,12-14,17H2,1-3H3,(H,33,38)(H,35,39)(H,36,40)/t19-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50602863

(CHEMBL1997924)Show SMILES C(Nc1nc(Nc2cc([nH]n2)C2CC2)c2sccc2n1)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114054
BindingDB Entry DOI: 10.7270/Q20K2DM8 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50325983

(6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidaz...)Show SMILES Cc1c[nH]c(n1)-c1cnc(NCCNc2ccc(cn2)C#N)nc1-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C22H18Cl2N8/c1-13-10-29-21(31-13)17-12-30-22(32-20(17)16-4-3-15(23)8-18(16)24)27-7-6-26-19-5-2-14(9-25)11-28-19/h2-5,8,10-12H,6-7H2,1H3,(H,26,28)(H,29,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114054
BindingDB Entry DOI: 10.7270/Q20K2DM8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50235342

(CHEMBL319467 | NU-6102)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(OCC3CCCCC3)c3nc[nH]c3n2)cc1 Show InChI InChI=1S/C18H22N6O3S/c19-28(25,26)14-8-6-13(7-9-14)22-18-23-16-15(20-11-21-16)17(24-18)27-10-12-4-2-1-3-5-12/h6-9,11-12H,1-5,10H2,(H2,19,25,26)(H2,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476967

(SARS-CoV-2 3CLP and CoV inhibitor 15g)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)c1c(Cl)cccc1Cl Show InChI InChI=1S/C32H36Cl2N4O7/c1-17(2)13-24(38-31(42)25-15-19-22(36-25)10-5-11-27(19)44-3)30(41)37-23(14-18-7-6-12-35-29(18)40)26(39)16-45-32(43)28-20(33)8-4-9-21(28)34/h4-5,8-11,15,17-18,23-24,36H,6-7,12-14,16H2,1-3H3,(H,35,40)(H,37,41)(H,38,42)/t18-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235346
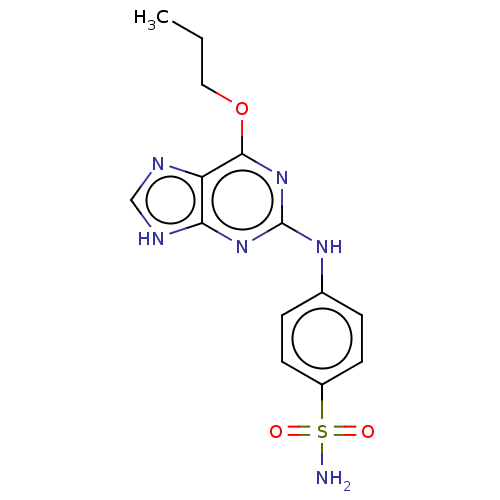
(CHEMBL4102518)Show SMILES CCCOc1nc(Nc2ccc(cc2)S(N)(=O)=O)nc2[nH]cnc12 Show InChI InChI=1S/C14H16N6O3S/c1-2-7-23-13-11-12(17-8-16-11)19-14(20-13)18-9-3-5-10(6-4-9)24(15,21)22/h3-6,8H,2,7H2,1H3,(H2,15,21,22)(H2,16,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged CDK2/bovine His-tagged Cyclin A |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50594616

(CHEMBL5170425)Show SMILES COc1cc(Cl)cc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50602863

(CHEMBL1997924)Show SMILES C(Nc1nc(Nc2cc([nH]n2)C2CC2)c2sccc2n1)c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114054
BindingDB Entry DOI: 10.7270/Q20K2DM8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek2
(Homo sapiens (Human)) | BDBM50553217

(CHEMBL4761747)Show SMILES CN(C)S(=O)(=O)Cc1cccc(Nc2nc(C#C)c3nc[nH]c3n2)c1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length His-tagged NEK2 expressed in baculovirus expression system assessed as inhibition of substrate phosphoryl... |
Citation and Details
Article DOI: 10.1039/d0md00074d
BindingDB Entry DOI: 10.7270/Q2P272RV |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM640398
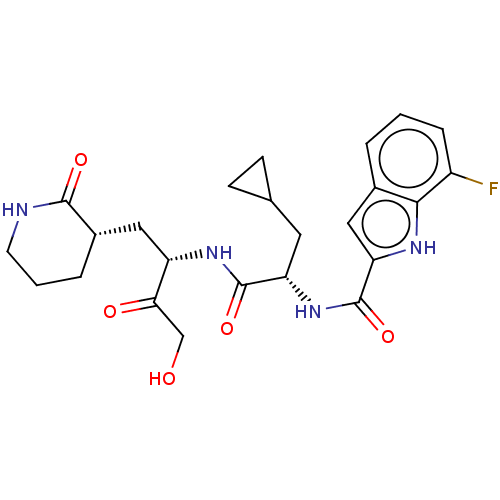
(Synthesis of N-[(2S)-3-cyclopropyl-1-({(2S)-4-hydr...)Show SMILES OCC(=O)[C@H](C[C@@H]1CCCNC1=O)NC(=O)[C@H](CC1CC1)NC(=O)c1cc2cccc(F)c2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM640396
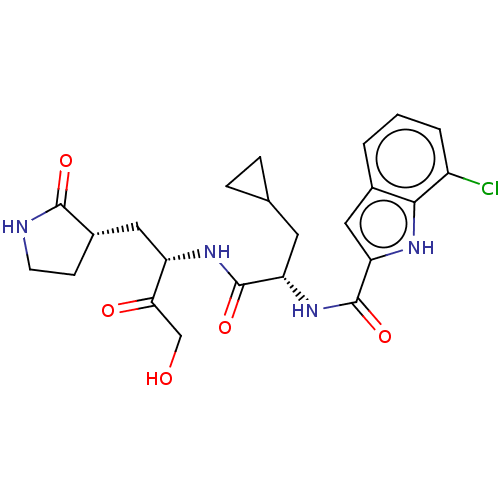
(Synthesis of 7-chloro-N-[(2S)-3-cyclopropyl-1-({(2...)Show SMILES OCC(=O)[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC1CC1)NC(=O)c1cc2cccc(Cl)c2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM640395
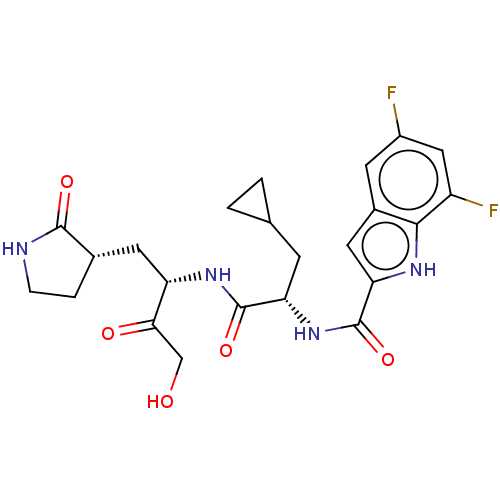
(Synthesis of N-[(2S)-3-cyclopropyl-1-({(2S)-4-hydr...)Show SMILES OCC(=O)[C@H](C[C@@H]1CCNC1=O)NC(=O)[C@H](CC1CC1)NC(=O)c1cc2cc(F)cc(F)c2[nH]1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM228619
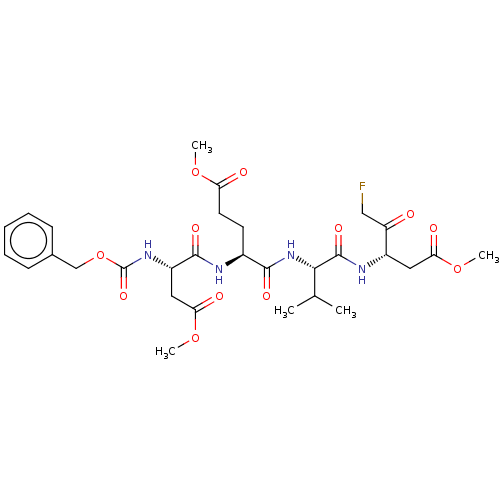
(US9345789, Z-DEVD-FMK)Show SMILES COC(=O)CC[C@H](NC(=O)[C@H](CC(=O)OC)NC(=O)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)OC)C(=O)CF Show InChI InChI=1S/C30H41FN4O12/c1-17(2)26(29(42)33-20(22(36)15-31)13-24(38)45-4)35-27(40)19(11-12-23(37)44-3)32-28(41)21(14-25(39)46-5)34-30(43)47-16-18-9-7-6-8-10-18/h6-10,17,19-21,26H,11-16H2,1-5H3,(H,32,41)(H,33,42)(H,34,43)(H,35,40)/t19-,20-,21-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM46060
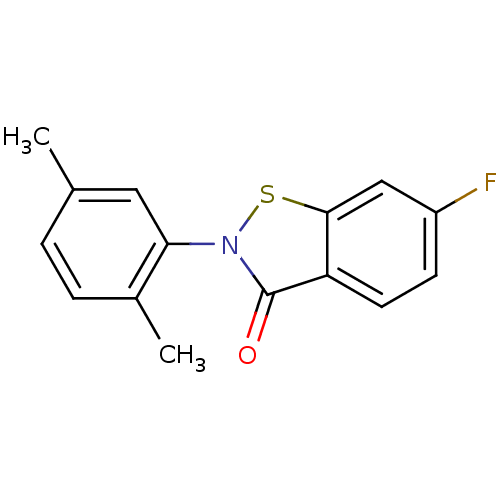
(2-(2,5-dimethylphenyl)-6-fluoranyl-1,2-benzothiazo...)Show InChI InChI=1S/C15H12FNOS/c1-9-3-4-10(2)13(7-9)17-15(18)12-6-5-11(16)8-14(12)19-17/h3-8H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM228619

(US9345789, Z-DEVD-FMK)Show SMILES COC(=O)CC[C@H](NC(=O)[C@H](CC(=O)OC)NC(=O)OCc1ccccc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(=O)OC)C(=O)CF Show InChI InChI=1S/C30H41FN4O12/c1-17(2)26(29(42)33-20(22(36)15-31)13-24(38)45-4)35-27(40)19(11-12-23(37)44-3)32-28(41)21(14-25(39)46-5)34-30(43)47-16-18-9-7-6-8-10-18/h6-10,17,19-21,26H,11-16H2,1-5H3,(H,32,41)(H,33,42)(H,34,43)(H,35,40)/t19-,20-,21-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek2
(Homo sapiens (Human)) | BDBM50553212
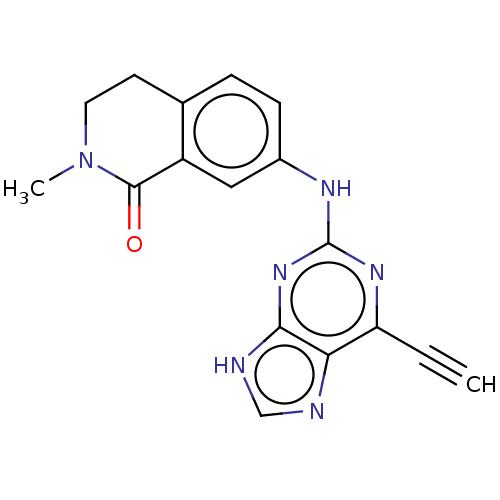
(CHEMBL4763380) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length His-tagged NEK2 expressed in baculovirus expression system assessed as inhibition of substrate phosphoryl... |
Citation and Details
Article DOI: 10.1039/d0md00074d
BindingDB Entry DOI: 10.7270/Q2P272RV |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235347

(CHEMBL4078246)Show SMILES CC(C)Oc1nc(Nc2ccc(cc2)S(N)(=O)=O)nc2[nH]cnc12 Show InChI InChI=1S/C14H16N6O3S/c1-8(2)23-13-11-12(17-7-16-11)19-14(20-13)18-9-3-5-10(6-4-9)24(15,21)22/h3-8H,1-2H3,(H2,15,21,22)(H2,16,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged CDK2/bovine His-tagged Cyclin A |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50308060
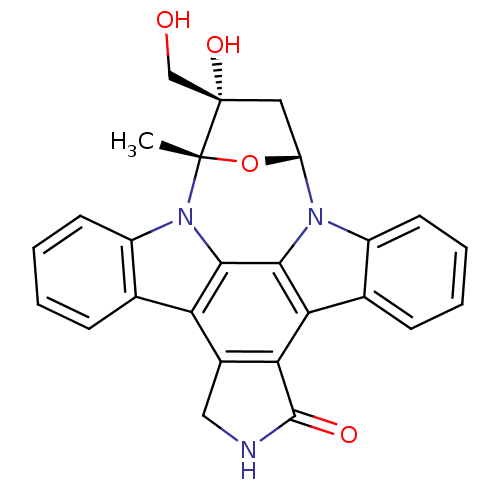
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114054
BindingDB Entry DOI: 10.7270/Q20K2DM8 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476964
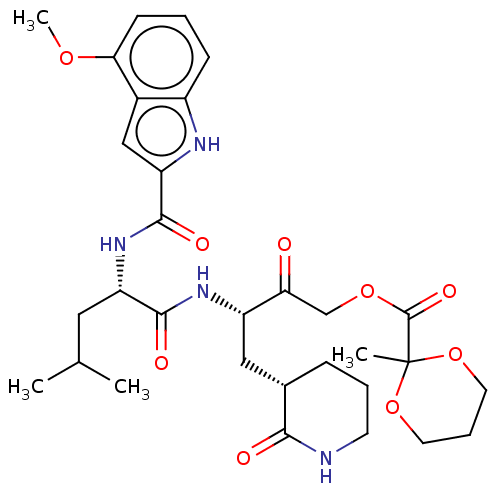
(SARS-CoV-2 3CLP and CoV inhibitor 15d)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)C1(C)OCCCO1 Show InChI InChI=1S/C31H42N4O9/c1-18(2)14-23(35-29(39)24-16-20-21(33-24)9-5-10-26(20)41-4)28(38)34-22(15-19-8-6-11-32-27(19)37)25(36)17-42-30(40)31(3)43-12-7-13-44-31/h5,9-10,16,18-19,22-23,33H,6-8,11-15,17H2,1-4H3,(H,32,37)(H,34,38)(H,35,39)/t19-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50594612
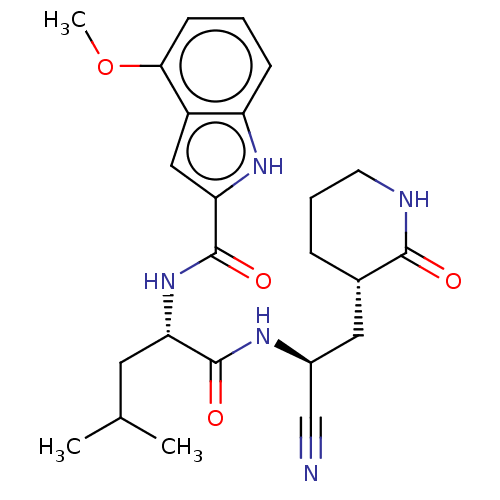
(CHEMBL5185160)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM582824
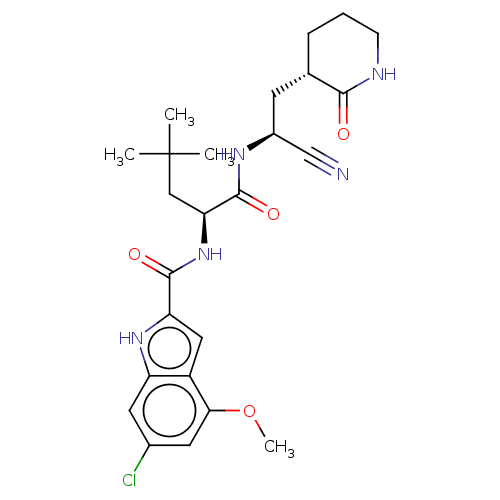
(US11524940, Compound 1149)Show SMILES COc1cc(Cl)cc2[nH]c(cc12)C(=O)N[C@@H](CC(C)(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50594614
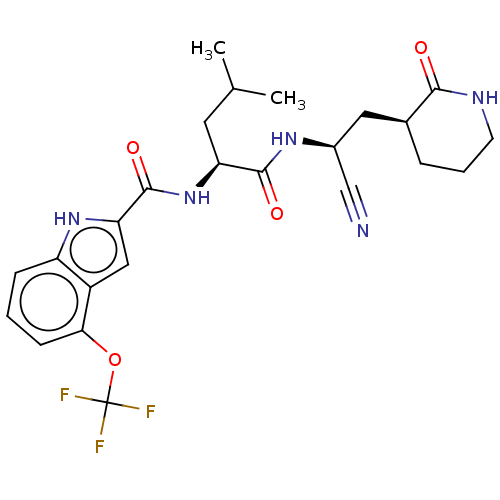
(CHEMBL5190756)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(OC(F)(F)F)cccc2[nH]1)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476973
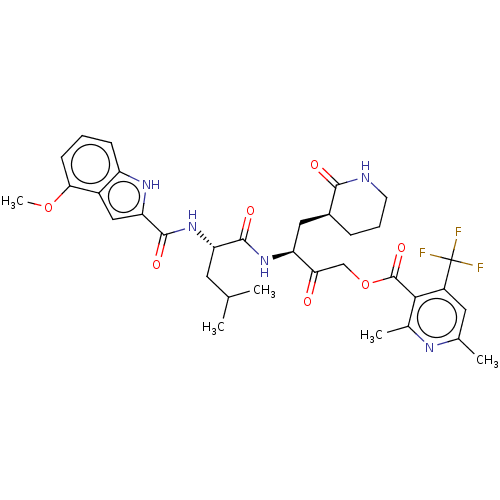
(SARS-CoV-2 3CLP and CoV inhibitor 15m)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)c1c(C)nc(C)cc1C(F)(F)F Show InChI InChI=1S/C34H40F3N5O7/c1-17(2)12-25(42-32(46)26-15-21-23(40-26)9-6-10-28(21)48-5)31(45)41-24(14-20-8-7-11-38-30(20)44)27(43)16-49-33(47)29-19(4)39-18(3)13-22(29)34(35,36)37/h6,9-10,13,15,17,20,24-25,40H,7-8,11-12,14,16H2,1-5H3,(H,38,44)(H,41,45)(H,42,46)/t20-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50233206
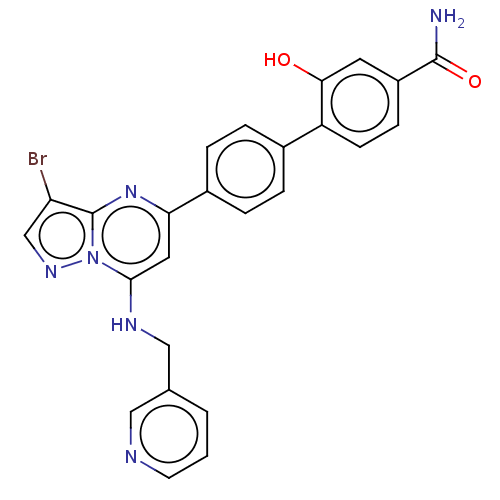
(CHEMBL4059895)Show SMILES NC(=O)c1ccc(c(O)c1)-c1ccc(cc1)-c1cc(NCc2cccnc2)n2ncc(Br)c2n1 Show InChI InChI=1S/C25H19BrN6O2/c26-20-14-30-32-23(29-13-15-2-1-9-28-12-15)11-21(31-25(20)32)17-5-3-16(4-6-17)19-8-7-18(24(27)34)10-22(19)33/h1-12,14,29,33H,13H2,(H2,27,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Masaryk University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E (unknown origin) expressed in baculovirus infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Eur J Med Chem 126: 1118-1128 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.023
BindingDB Entry DOI: 10.7270/Q29C70PT |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235349
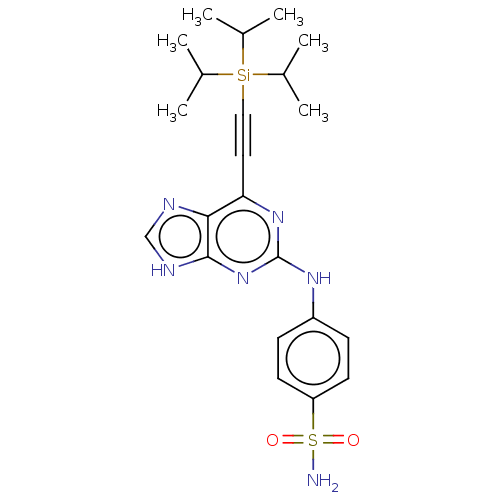
(CHEMBL4065402)Show SMILES CC(C)[Si](C#Cc1nc(Nc2ccc(cc2)S(N)(=O)=O)nc2[nH]cnc12)(C(C)C)C(C)C Show InChI InChI=1S/C22H30N6O2SSi/c1-14(2)32(15(3)4,16(5)6)12-11-19-20-21(25-13-24-20)28-22(27-19)26-17-7-9-18(10-8-17)31(23,29)30/h7-10,13-16H,1-6H3,(H2,23,29,30)(H2,24,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
In vitro inhibition against angiotensin converting enzyme (ACE) |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235336
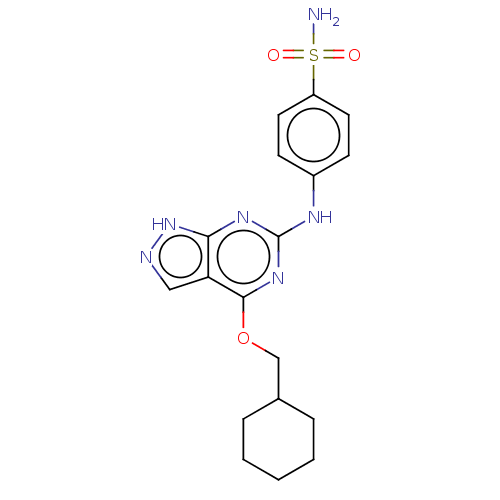
(CHEMBL4085238)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(OCC3CCCCC3)c3cn[nH]c3n2)cc1 Show InChI InChI=1S/C18H22N6O3S/c19-28(25,26)14-8-6-13(7-9-14)21-18-22-16-15(10-20-24-16)17(23-18)27-11-12-4-2-1-3-5-12/h6-10,12H,1-5,11H2,(H2,19,25,26)(H2,20,21,22,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM50233212
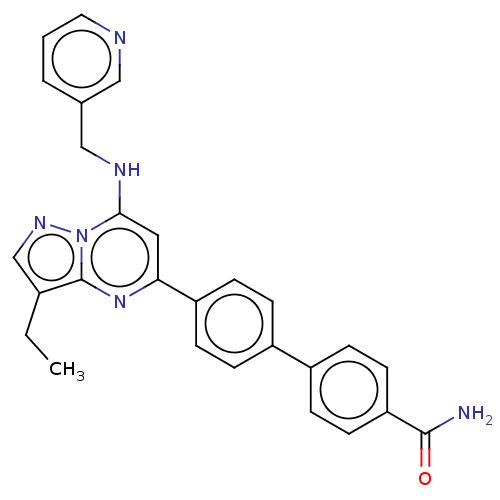
(CHEMBL4076762)Show SMILES CCc1cnn2c(NCc3cccnc3)cc(nc12)-c1ccc(cc1)-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C27H24N6O/c1-2-19-17-31-33-25(30-16-18-4-3-13-29-15-18)14-24(32-27(19)33)22-9-5-20(6-10-22)21-7-11-23(12-8-21)26(28)34/h3-15,17,30H,2,16H2,1H3,(H2,28,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Masaryk University
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/cyclin E (unknown origin) expressed in baculovirus infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Eur J Med Chem 126: 1118-1128 (2017)
Article DOI: 10.1016/j.ejmech.2016.12.023
BindingDB Entry DOI: 10.7270/Q29C70PT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek2
(Homo sapiens (Human)) | BDBM50553207
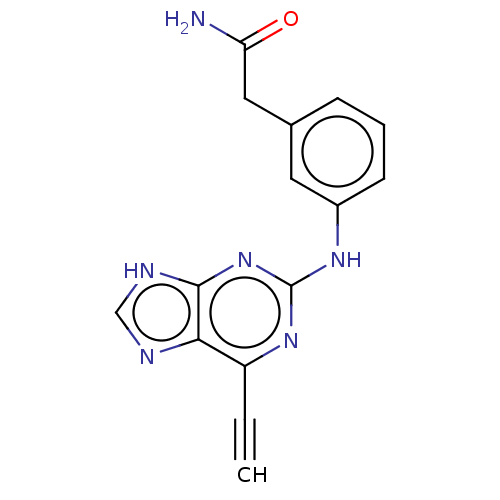
(CHEMBL4786783) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NEK2 by kinase-profiling analysis |
Citation and Details
Article DOI: 10.1039/d0md00074d
BindingDB Entry DOI: 10.7270/Q2P272RV |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235344
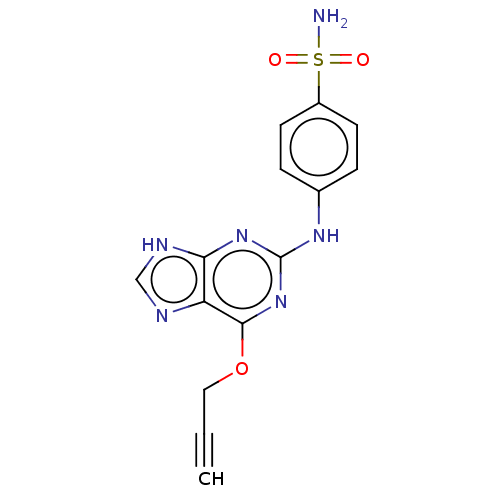
(CHEMBL4086832)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(OCC#C)c3nc[nH]c3n2)cc1 Show InChI InChI=1S/C14H12N6O3S/c1-2-7-23-13-11-12(17-8-16-11)19-14(20-13)18-9-3-5-10(6-4-9)24(15,21)22/h1,3-6,8H,7H2,(H2,15,21,22)(H2,16,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
In vitro inhibition against angiotensin converting enzyme (ACE) |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476972
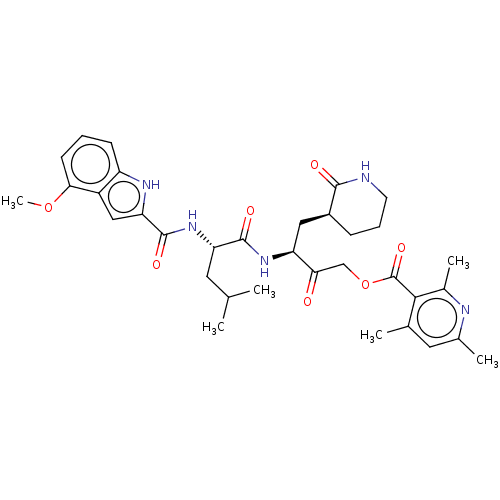
(SARS-CoV-2 3CLP and CoV inhibitor 15l)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)c1c(C)cc(C)nc1C Show InChI InChI=1S/C34H43N5O7/c1-18(2)13-26(39-33(43)27-16-23-24(37-27)10-7-11-29(23)45-6)32(42)38-25(15-22-9-8-12-35-31(22)41)28(40)17-46-34(44)30-19(3)14-20(4)36-21(30)5/h7,10-11,14,16,18,22,25-26,37H,8-9,12-13,15,17H2,1-6H3,(H,35,41)(H,38,42)(H,39,43)/t22-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235354
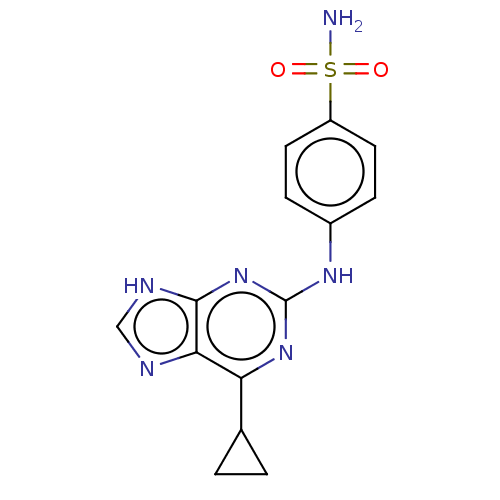
(CHEMBL4069161)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(C3CC3)c3nc[nH]c3n2)cc1 Show InChI InChI=1S/C14H14N6O2S/c15-23(21,22)10-5-3-9(4-6-10)18-14-19-11(8-1-2-8)12-13(20-14)17-7-16-12/h3-8H,1-2H2,(H2,15,21,22)(H2,16,17,18,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM46060

(2-(2,5-dimethylphenyl)-6-fluoranyl-1,2-benzothiazo...)Show InChI InChI=1S/C15H12FNOS/c1-9-3-4-10(2)13(7-9)17-15(18)12-6-5-11(16)8-14(12)19-17/h3-8H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476959

(SARS-CoV-2 3CLP and CoV inhibitor 5)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)CO Show InChI InChI=1S/C25H34N4O6/c1-14(2)10-19(29-25(34)20-12-16-17(27-20)7-4-8-22(16)35-3)24(33)28-18(21(31)13-30)11-15-6-5-9-26-23(15)32/h4,7-8,12,14-15,18-19,27,30H,5-6,9-11,13H2,1-3H3,(H,26,32)(H,28,33)(H,29,34)/t15-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476963

(SARS-CoV-2 3CLP and CoV inhibitor 15c)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)C(C)(C)C(F)(F)F Show InChI InChI=1S/C30H39F3N4O7/c1-16(2)12-21(37-27(41)22-14-18-19(35-22)9-6-10-24(18)43-5)26(40)36-20(13-17-8-7-11-34-25(17)39)23(38)15-44-28(42)29(3,4)30(31,32)33/h6,9-10,14,16-17,20-21,35H,7-8,11-13,15H2,1-5H3,(H,34,39)(H,36,40)(H,37,41)/t17-,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476959

(SARS-CoV-2 3CLP and CoV inhibitor 5)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)CO Show InChI InChI=1S/C25H34N4O6/c1-14(2)10-19(29-25(34)20-12-16-17(27-20)7-4-8-22(16)35-3)24(33)28-18(21(31)13-30)11-15-6-5-9-26-23(15)32/h4,7-8,12,14-15,18-19,27,30H,5-6,9-11,13H2,1-3H3,(H,26,32)(H,28,33)(H,29,34)/t15-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fraunhofer Institute for Translational Medicine and Pharmacology (ITMP) and Fraunhofer Cluster of Excellence for Immune mediated diseases (CIMD)
| Assay Description
Primary assay principle based on quenched FRET peptide substrate of SARS-CoV-2 3CL-Pro (lhs). Inhibiting compounds reduce fluorescence signal relativ... |
bioRxiv 2020: (2020)
Article DOI: 10.1101/2020.12.16.422677
BindingDB Entry DOI: 10.7270/Q26M39VR |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476974

(SARS-CoV-2 3CLP and CoV inhibitor 15n)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)c1c(C)nc(C)nc1C Show InChI InChI=1S/C33H42N6O7/c1-17(2)13-25(39-32(43)26-15-22-23(37-26)10-7-11-28(22)45-6)31(42)38-24(14-21-9-8-12-34-30(21)41)27(40)16-46-33(44)29-18(3)35-20(5)36-19(29)4/h7,10-11,15,17,21,24-25,37H,8-9,12-14,16H2,1-6H3,(H,34,41)(H,38,42)(H,39,43)/t21-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM476962
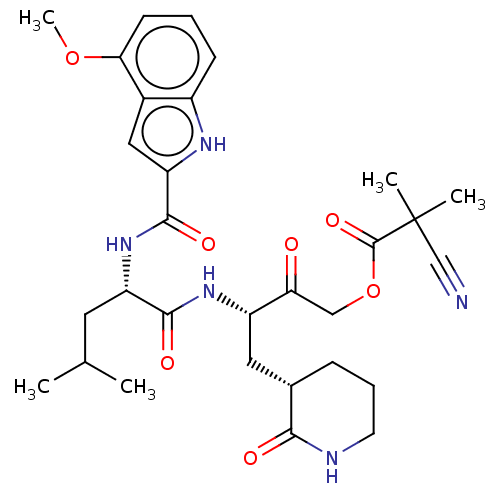
(SARS-CoV-2 3CLP and CoV inhibitor 15b)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C(=O)COC(=O)C(C)(C)C#N Show InChI InChI=1S/C30H39N5O7/c1-17(2)12-22(35-28(39)23-14-19-20(33-23)9-6-10-25(19)41-5)27(38)34-21(13-18-8-7-11-32-26(18)37)24(36)15-42-29(40)30(3,4)16-31/h6,9-10,14,17-18,21-22,33H,7-8,11-13,15H2,1-5H3,(H,32,37)(H,34,38)(H,35,39)/t18-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
| Assay Description
Pure SARS-CoV-2 3CLP was obtained as previously described in detail, and the enzymatic activity was confirmed according to an established protocol; s... |
J Med Chem (2021)
Article DOI: 10.1021/acs.jmedchem.1c00616
BindingDB Entry DOI: 10.7270/Q2BP05WB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM419133

(BDBM429386 | GC376)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(O)S([O-])(=O)=O Show InChI InChI=1S/C21H31N3O8S/c1-13(2)10-16(24-21(28)32-12-14-6-4-3-5-7-14)19(26)23-17(20(27)33(29,30)31)11-15-8-9-22-18(15)25/h3-7,13,15-17,20,27H,8-12H2,1-2H3,(H,22,25)(H,23,26)(H,24,28)(H,29,30,31)/p-1/t15?,16-,17-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50594615

(CHEMBL5204224)Show SMILES CCOc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCCNC1=O)C#N |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d1md00247c
BindingDB Entry DOI: 10.7270/Q2HH6Q3R |
More data for this
Ligand-Target Pair | |
Cyclin-A2
(Bos taurus) | BDBM50235345
(CHEMBL4105278)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(-c3ccccc3)c3nc[nH]c3n2)cc1 Show InChI InChI=1S/C17H14N6O2S/c18-26(24,25)13-8-6-12(7-9-13)21-17-22-14(11-4-2-1-3-5-11)15-16(23-17)20-10-19-15/h1-10H,(H2,18,24,25)(H2,19,20,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against angiotensin-converting enzyme |
J Med Chem 60: 1746-1767 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01254
BindingDB Entry DOI: 10.7270/Q2833V86 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data