 Found 112 hits with Last Name = 'campo' and Initial = 'b'
Found 112 hits with Last Name = 'campo' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009661
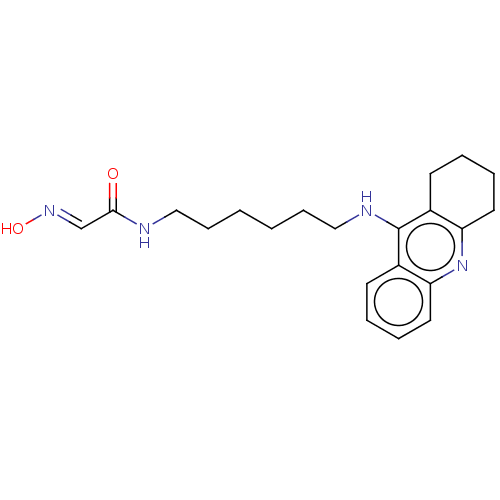
(CHEMBL3235218)Show InChI InChI=1S/C21H28N4O2/c26-20(15-24-27)22-13-7-1-2-8-14-23-21-16-9-3-5-11-18(16)25-19-12-6-4-10-17(19)21/h3,5,9,11,15,27H,1-2,4,6-8,10,12-14H2,(H,22,26)(H,23,25)/b24-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 588 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009659

(CHEMBL3235216)Show InChI InChI=1S/C19H24N4O2/c24-18(13-22-25)20-11-5-6-12-21-19-14-7-1-3-9-16(14)23-17-10-4-2-8-15(17)19/h1,3,7,9,13,25H,2,4-6,8,10-12H2,(H,20,24)(H,21,23)/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009660
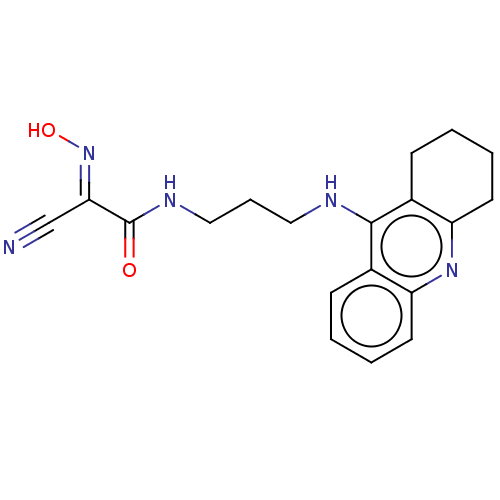
(CHEMBL3235217)Show InChI InChI=1S/C19H21N5O2/c20-12-17(24-26)19(25)22-11-5-10-21-18-13-6-1-3-8-15(13)23-16-9-4-2-7-14(16)18/h1,3,6,8,26H,2,4-5,7,9-11H2,(H,21,23)(H,22,25)/b24-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009658
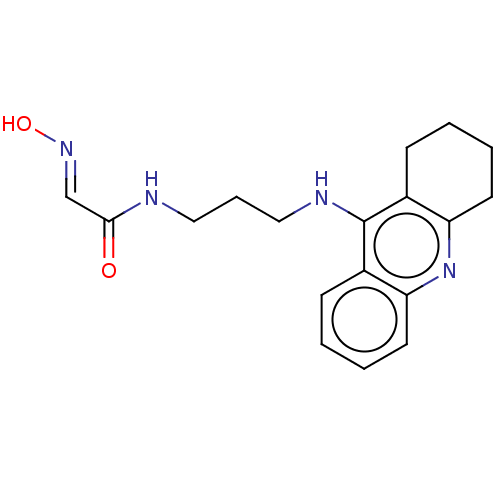
(CHEMBL3235215)Show InChI InChI=1S/C18H22N4O2.ClH/c23-17(12-21-24)19-10-5-11-20-18-13-6-1-3-8-15(13)22-16-9-4-2-7-14(16)18;/h1,3,6,8,12,24H,2,4-5,7,9-11H2,(H,19,23)(H,20,22);1H/b21-12+; | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009664
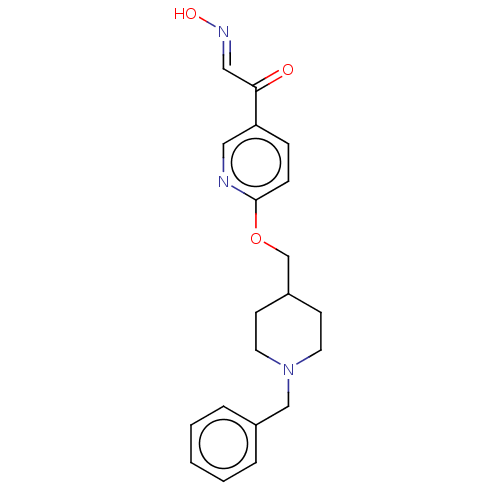
(CHEMBL3235221)Show InChI InChI=1S/C20H23N3O3/c24-19(13-22-25)18-6-7-20(21-12-18)26-15-17-8-10-23(11-9-17)14-16-4-2-1-3-5-16/h1-7,12-13,17,25H,8-11,14-15H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009670

(CHEMBL3235227)Show InChI InChI=1S/C19H22N4O3/c24-18(12-22-25)17-10-20-19(21-11-17)26-14-16-6-8-23(9-7-16)13-15-4-2-1-3-5-15/h1-5,10-12,16,25H,6-9,13-14H2/b22-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009694
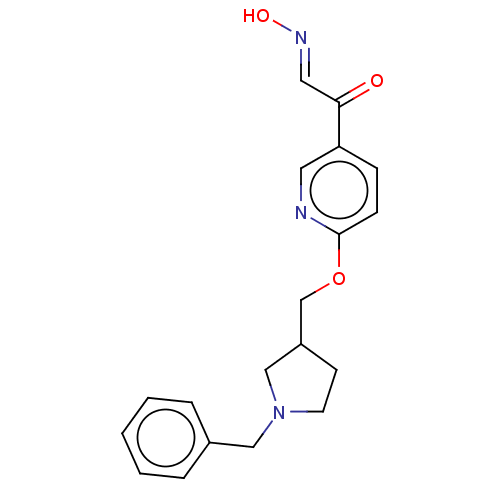
(CHEMBL3235232)Show InChI InChI=1S/C19H21N3O3/c23-18(11-21-24)17-6-7-19(20-10-17)25-14-16-8-9-22(13-16)12-15-4-2-1-3-5-15/h1-7,10-11,16,24H,8-9,12-14H2/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009663

(CHEMBL3235220)Show InChI InChI=1S/C20H23N3O3/c24-19(13-22-25)18-6-9-21-20(12-18)26-15-17-7-10-23(11-8-17)14-16-4-2-1-3-5-16/h1-6,9,12-13,17,25H,7-8,10-11,14-15H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009667
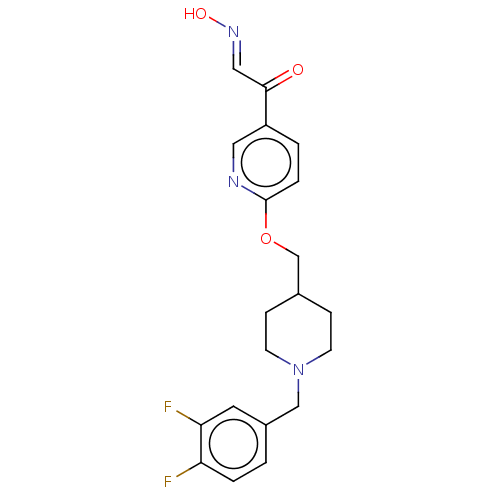
(CHEMBL3235224)Show SMILES O\N=C\C(=O)c1ccc(OCC2CCN(Cc3ccc(F)c(F)c3)CC2)nc1 Show InChI InChI=1S/C20H21F2N3O3/c21-17-3-1-15(9-18(17)22)12-25-7-5-14(6-8-25)13-28-20-4-2-16(10-23-20)19(26)11-24-27/h1-4,9-11,14,27H,5-8,12-13H2/b24-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009662

(CHEMBL3235219 | US9162983, C)Show InChI InChI=1S/C20H23N3O3/c24-19(13-22-25)18-7-4-10-21-20(18)26-15-17-8-11-23(12-9-17)14-16-5-2-1-3-6-16/h1-7,10,13,17,25H,8-9,11-12,14-15H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009715
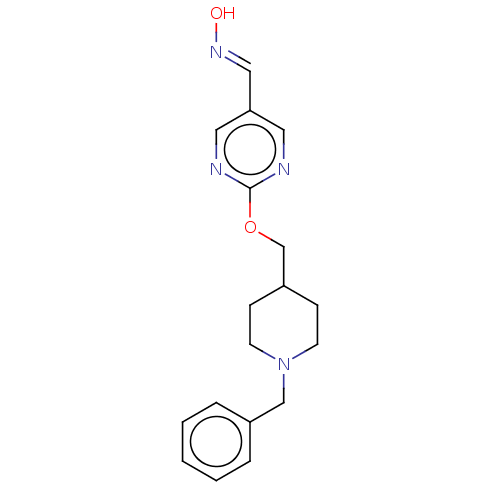
(CHEMBL3235235)Show InChI InChI=1S/C18H22N4O2/c23-21-12-17-10-19-18(20-11-17)24-14-16-6-8-22(9-7-16)13-15-4-2-1-3-5-15/h1-5,10-12,16,23H,6-9,13-14H2/b21-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009665
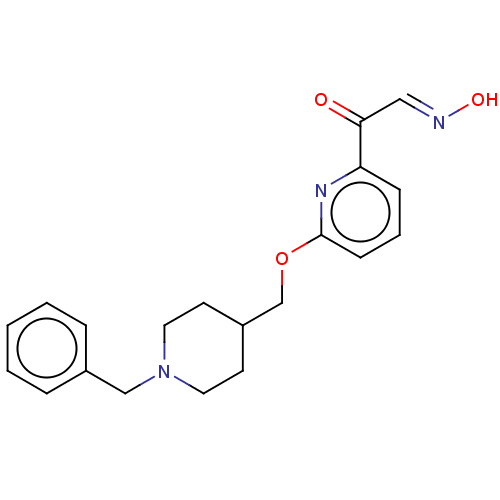
(CHEMBL3235222)Show InChI InChI=1S/C20H23N3O3/c24-19(13-21-25)18-7-4-8-20(22-18)26-15-17-9-11-23(12-10-17)14-16-5-2-1-3-6-16/h1-8,13,17,25H,9-12,14-15H2/b21-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009675
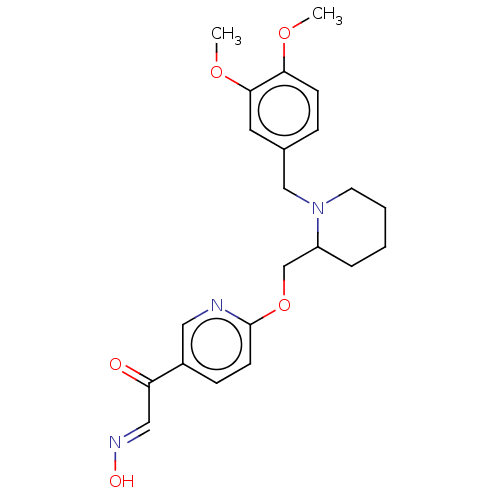
(CHEMBL3235230)Show SMILES COc1ccc(CN2CCCCC2COc2ccc(cn2)C(=O)\C=N\O)cc1OC Show InChI InChI=1S/C22H27N3O5/c1-28-20-8-6-16(11-21(20)29-2)14-25-10-4-3-5-18(25)15-30-22-9-7-17(12-23-22)19(26)13-24-27/h6-9,11-13,18,27H,3-5,10,14-15H2,1-2H3/b24-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009650

(CHEMBL3235207)Show InChI InChI=1S/C16H21N3O3/c1-12(20)15(18-22)16(21)17-14-7-9-19(10-8-14)11-13-5-3-2-4-6-13/h2-6,14,22H,7-11H2,1H3,(H,17,21)/b18-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009713
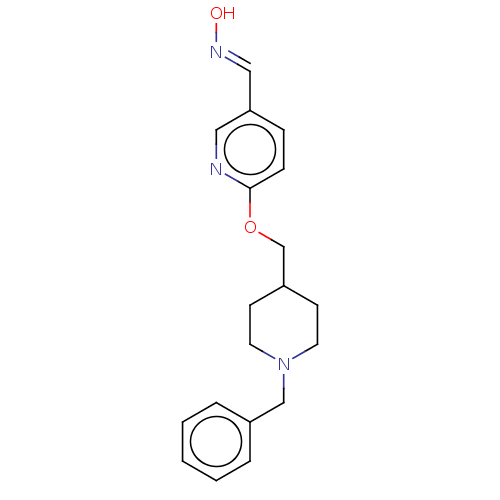
(CHEMBL3235234)Show InChI InChI=1S/C19H23N3O2/c23-21-13-18-6-7-19(20-12-18)24-15-17-8-10-22(11-9-17)14-16-4-2-1-3-5-16/h1-7,12-13,17,23H,8-11,14-15H2/b21-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.66E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009671
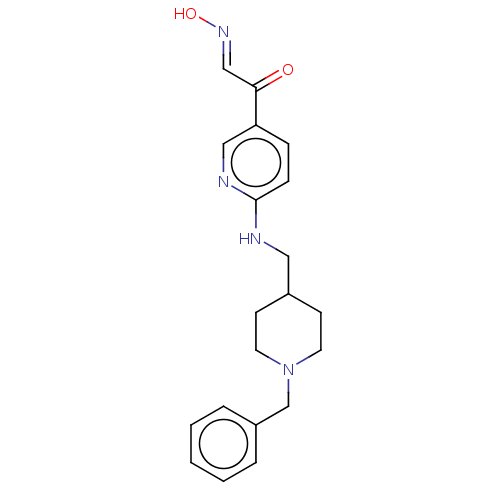
(CHEMBL3235228)Show InChI InChI=1S/C20H24N4O2/c25-19(14-23-26)18-6-7-20(22-13-18)21-12-16-8-10-24(11-9-16)15-17-4-2-1-3-5-17/h1-7,13-14,16,26H,8-12,15H2,(H,21,22)/b23-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009669

(CHEMBL3235226)Show SMILES O\N=C\C(=O)c1ccc(OCC2CCN(Cc3cccc(Cl)c3)CC2)nc1 Show InChI InChI=1S/C20H22ClN3O3/c21-18-3-1-2-16(10-18)13-24-8-6-15(7-9-24)14-27-20-5-4-17(11-22-20)19(25)12-23-26/h1-5,10-12,15,26H,6-9,13-14H2/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009673
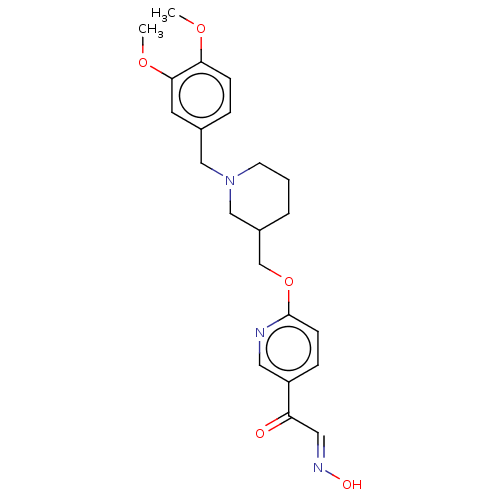
(CHEMBL3235229)Show SMILES COc1ccc(CN2CCCC(COc3ccc(cn3)C(=O)\C=N\O)C2)cc1OC Show InChI InChI=1S/C22H27N3O5/c1-28-20-7-5-16(10-21(20)29-2)13-25-9-3-4-17(14-25)15-30-22-8-6-18(11-23-22)19(26)12-24-27/h5-8,10-12,17,27H,3-4,9,13-15H2,1-2H3/b24-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009692

(CHEMBL3235231)Show InChI InChI=1S/C20H23N3O3/c24-19(13-22-25)17-9-10-20(21-12-17)26-15-18-8-4-5-11-23(18)14-16-6-2-1-3-7-16/h1-3,6-7,9-10,12-13,18,25H,4-5,8,11,14-15H2/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009655
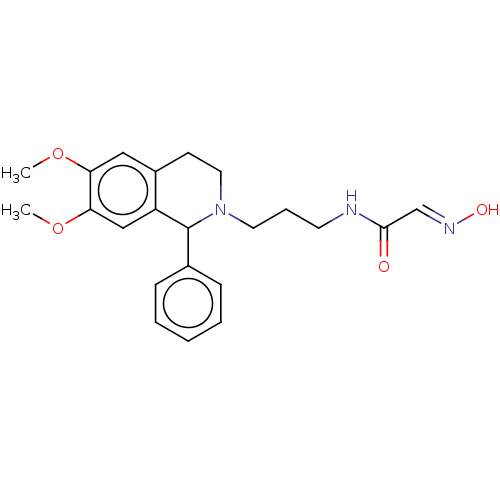
(CHEMBL3235212)Show SMILES COc1cc2CCN(CCCNC(=O)\C=N\O)C(c3ccccc3)c2cc1OC Show InChI InChI=1S/C22H27N3O4/c1-28-19-13-17-9-12-25(11-6-10-23-21(26)15-24-27)22(16-7-4-3-5-8-16)18(17)14-20(19)29-2/h3-5,7-8,13-15,22,27H,6,9-12H2,1-2H3,(H,23,26)/b24-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009652

(CHEMBL3235209)Show InChI InChI=1S/C15H18N4O2/c16-10-14(18-21)15(20)17-13-6-8-19(9-7-13)11-12-4-2-1-3-5-12/h1-5,13,21H,6-9,11H2,(H,17,20)/b18-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009668
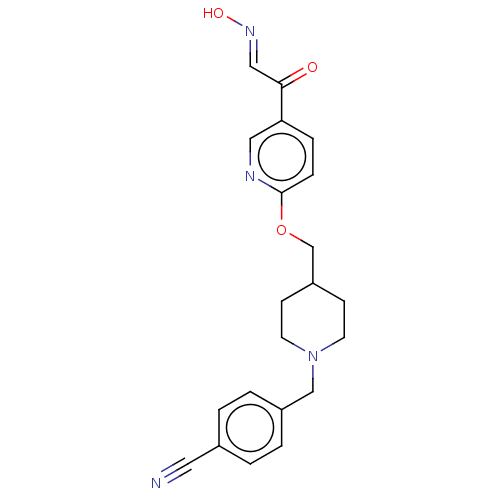
(CHEMBL3235225)Show SMILES O\N=C\C(=O)c1ccc(OCC2CCN(Cc3ccc(cc3)C#N)CC2)nc1 Show InChI InChI=1S/C21H22N4O3/c22-11-16-1-3-17(4-2-16)14-25-9-7-18(8-10-25)15-28-21-6-5-19(12-23-21)20(26)13-24-27/h1-6,12-13,18,27H,7-10,14-15H2/b24-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.61E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009654

(CHEMBL3235211)Show SMILES COc1ccc(CN2CCC(CNC(=O)C(=N\O)\C(C)=O)CC2)cc1OC Show InChI InChI=1S/C19H27N3O5/c1-13(23)18(21-25)19(24)20-11-14-6-8-22(9-7-14)12-15-4-5-16(26-2)17(10-15)27-3/h4-5,10,14,25H,6-9,11-12H2,1-3H3,(H,20,24)/b21-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.63E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009656

(CHEMBL3235213)Show SMILES COc1cc2CCN(CCCNC(=O)C(=N\O)\C(C)=O)C(c3ccccc3)c2cc1OC Show InChI InChI=1S/C24H29N3O5/c1-16(28)22(26-30)24(29)25-11-7-12-27-13-10-18-14-20(31-2)21(32-3)15-19(18)23(27)17-8-5-4-6-9-17/h4-6,8-9,14-15,23,30H,7,10-13H2,1-3H3,(H,25,29)/b26-22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009666

(CHEMBL3235223)Show SMILES COc1ccc(CN2CCC(COc3ccc(cn3)C(=O)\C=N\O)CC2)cc1OC Show InChI InChI=1S/C22H27N3O5/c1-28-20-5-3-17(11-21(20)29-2)14-25-9-7-16(8-10-25)15-30-22-6-4-18(12-23-22)19(26)13-24-27/h3-6,11-13,16,27H,7-10,14-15H2,1-2H3/b24-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.93E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009651
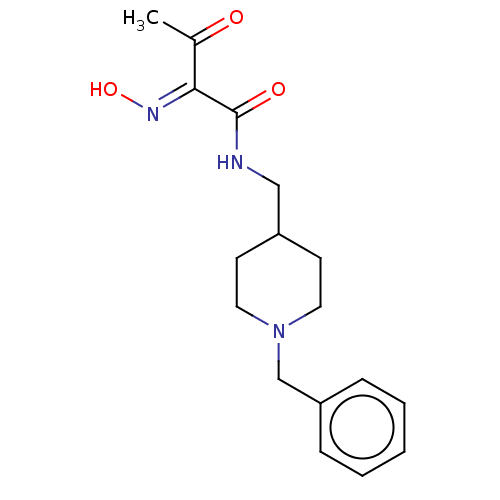
(CHEMBL3235208 | US9162983, E)Show InChI InChI=1S/C17H23N3O3/c1-13(21)16(19-23)17(22)18-11-14-7-9-20(10-8-14)12-15-5-3-2-4-6-15/h2-6,14,23H,7-12H2,1H3,(H,18,22)/b19-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009648

(CHEMBL3235205)Show InChI InChI=1S/C14H19N3O2/c18-14(10-15-19)16-13-6-8-17(9-7-13)11-12-4-2-1-3-5-12/h1-5,10,13,19H,6-9,11H2,(H,16,18)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.43E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009711
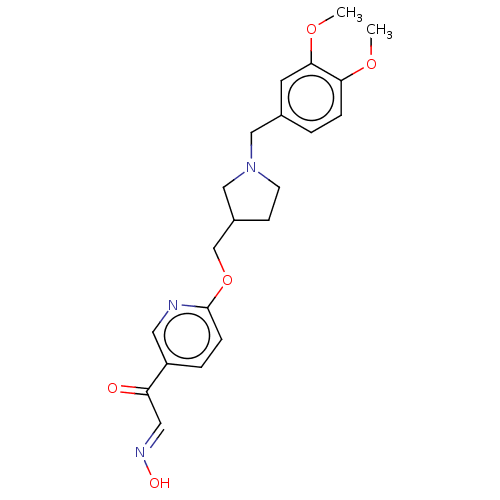
(CHEMBL3235233)Show SMILES COc1ccc(CN2CCC(COc3ccc(cn3)C(=O)\C=N\O)C2)cc1OC Show InChI InChI=1S/C21H25N3O5/c1-27-19-5-3-15(9-20(19)28-2)12-24-8-7-16(13-24)14-29-21-6-4-17(10-22-21)18(25)11-23-26/h3-6,9-11,16,26H,7-8,12-14H2,1-2H3/b23-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.52E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009657

(CHEMBL3235214)Show SMILES COc1cc2CCN(CCCNC(=O)C(=N\O)\C(C)=O)Cc2cc1OC Show InChI InChI=1S/C18H25N3O5/c1-12(22)17(20-24)18(23)19-6-4-7-21-8-5-13-9-15(25-2)16(26-3)10-14(13)11-21/h9-10,24H,4-8,11H2,1-3H3,(H,19,23)/b20-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.67E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009649

(CHEMBL3235206)Show InChI InChI=1S/C15H21N3O2/c19-15(11-17-20)16-10-13-6-8-18(9-7-13)12-14-4-2-1-3-5-14/h1-5,11,13,20H,6-10,12H2,(H,16,19)/b17-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50009653

(CHEMBL3235210)Show InChI InChI=1S/C16H20N4O2/c17-10-15(19-22)16(21)18-11-13-6-8-20(9-7-13)12-14-4-2-1-3-5-14/h1-5,13,22H,6-9,11-12H2,(H,18,21)/b19-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southwest Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate after 4 hrs by robotic spectrophotometric assay |
Bioorg Med Chem Lett 24: 1711-4 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.049
BindingDB Entry DOI: 10.7270/Q280545W |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603975

(CHEMBL5201780) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603974

(CHEMBL5201904)Show SMILES COc1ccc(cn1)-c1cnc2sc(N[C@@H]3CC[C@H](N)CC3)nn12 |r,wU:15.15,18.19,(-7.26,4.65,;-5.72,4.65,;-4.95,3.32,;-5.72,1.98,;-4.96,.65,;-3.42,.65,;-2.64,1.99,;-3.42,3.32,;-2.65,-.68,;-3.1,-2.13,;-1.86,-3.05,;-.65,-2.17,;.9,-2.18,;1.42,-.69,;2.91,-.3,;4,-1.38,;3.6,-2.87,;4.69,-3.96,;6.17,-3.56,;7.26,-4.65,;6.57,-2.07,;5.48,-.99,;.2,.22,;-1.11,-.68,)| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603992
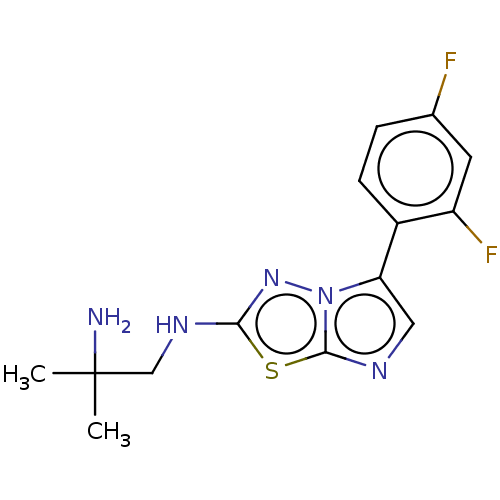
(CHEMBL5179988) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM151585

(US11739089, Compound Ketoconazole | US8987315, Ket...)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OCC2COC(Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes assessed as testosterone 6beta-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM151585

(US11739089, Compound Ketoconazole | US8987315, Ket...)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OCC2COC(Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes assessed as midazolam 1'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes assessed as dextromethorphan O-demethylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603978
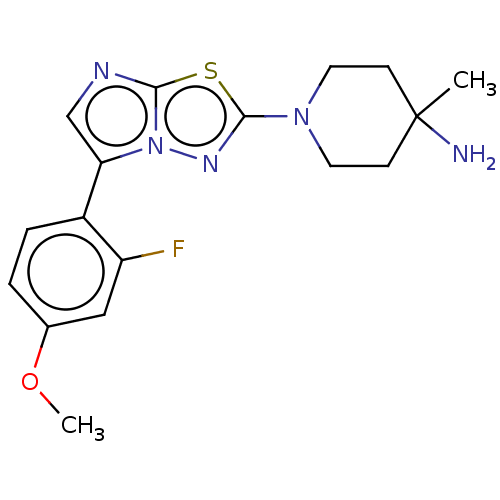
(CHEMBL5187817)Show SMILES COc1ccc(-c2cnc3sc(nn23)N2CCC(C)(N)CC2)c(F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603989
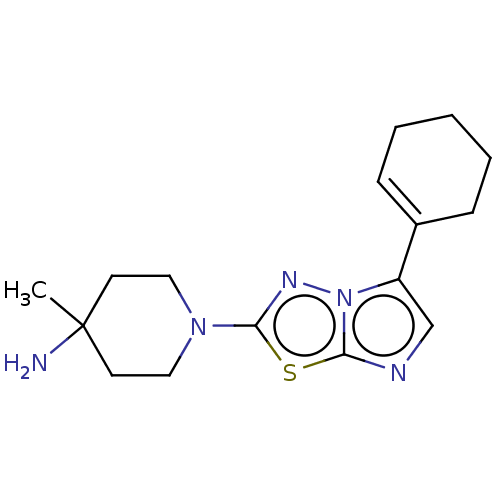
(CHEMBL5192443)Show SMILES CC1(N)CCN(CC1)c1nn2c(cnc2s1)C1=CCCCC1 |t:19| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603986

(CHEMBL5187610) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603988
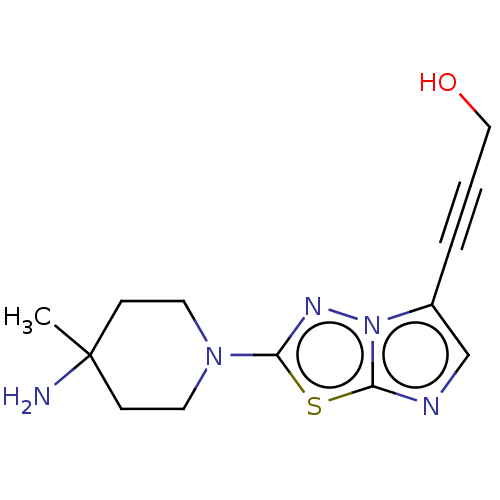
(CHEMBL5170360) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603984

(CHEMBL5195201)Show SMILES CC1(N)CCN(CC1)c1nn2c(cnc2s1)-c1cccc2cc[nH]c12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50273689
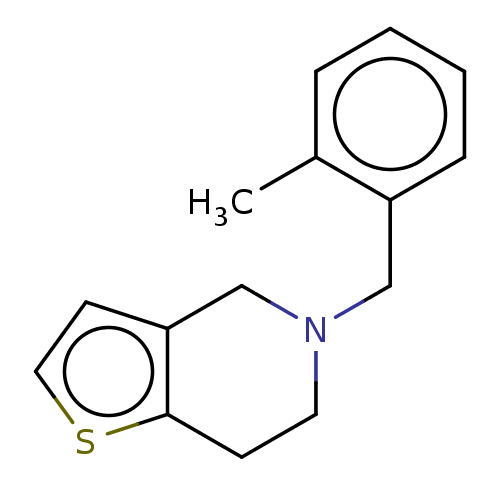
(CHEMBL4128999)Show InChI InChI=1S/C15H17NS/c1-12-4-2-3-5-13(12)10-16-8-6-15-14(11-16)7-9-17-15/h2-5,7,9H,6,8,10-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes assessed as (S)-mephenytoin 4'-hydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603985
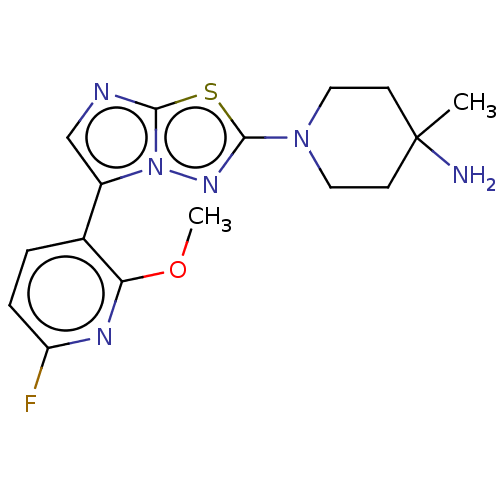
(CHEMBL5207627) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090677
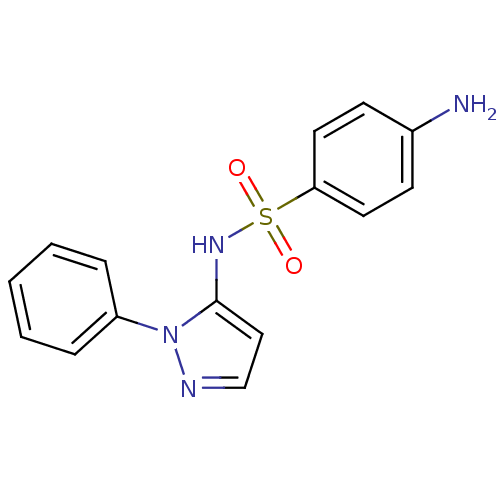
(4-Amino-N-(2-phenyl-2H-pyrazol-3-yl)-benzenesulfon...)Show InChI InChI=1S/C15H14N4O2S/c16-12-6-8-14(9-7-12)22(20,21)18-15-10-11-17-19(15)13-4-2-1-3-5-13/h1-11,18H,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Liverpool
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes assessed as tolbutamide methylhydroxylation after 4 to 40 mins in presence of NADPH by LCMS analysis |
Bioorg Med Chem 26: 2996-3005 (2018)
Article DOI: 10.1016/j.bmc.2018.05.006
BindingDB Entry DOI: 10.7270/Q2K076SB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603979
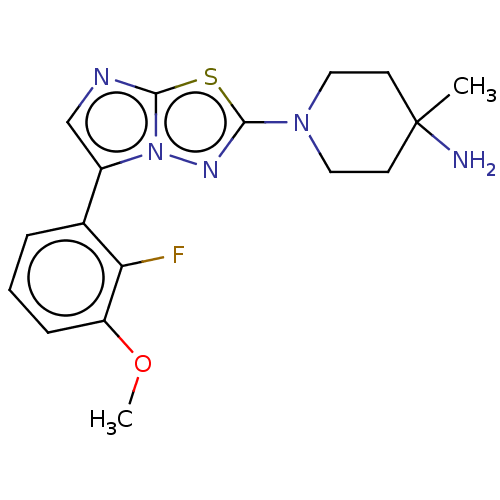
(CHEMBL5204715) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603976

(CHEMBL5200247) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603983

(CHEMBL5181699) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase haspin
(Homo sapiens (Human)) | BDBM50603977
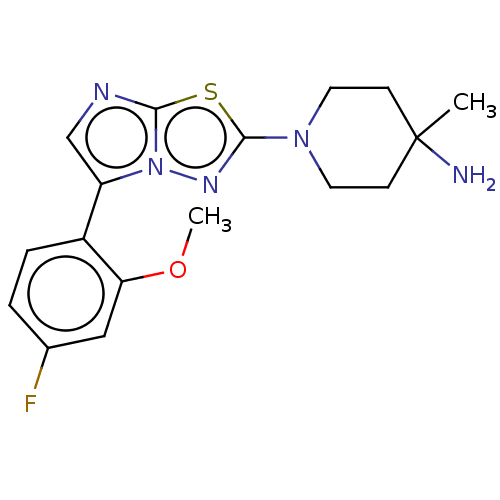
(CHEMBL5189523) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50603977

(CHEMBL5189523) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01995
BindingDB Entry DOI: 10.7270/Q2H70KXT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data