

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242974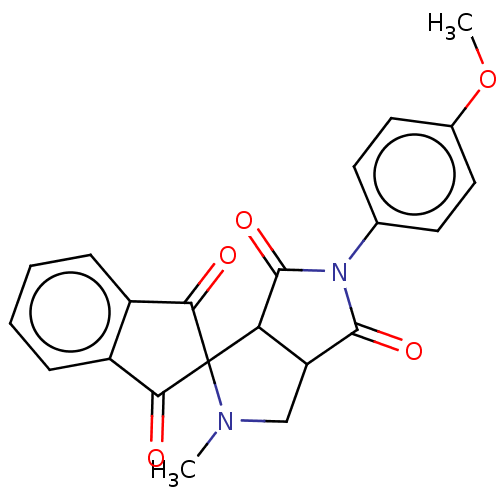 (CHEMBL4072825) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558500 (CHEMBL4752440) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558514 (CHEMBL4747183) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242995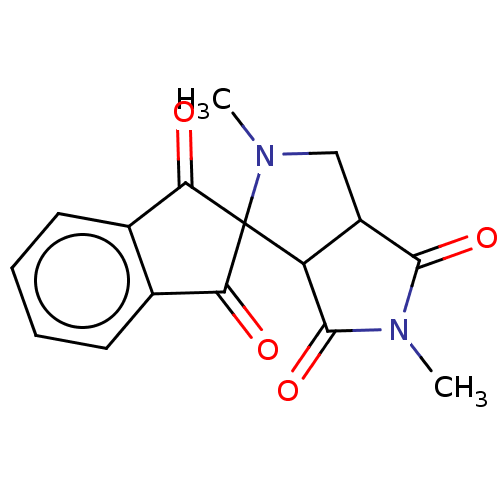 (CHEMBL4088091) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558508 (CHEMBL4794820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242971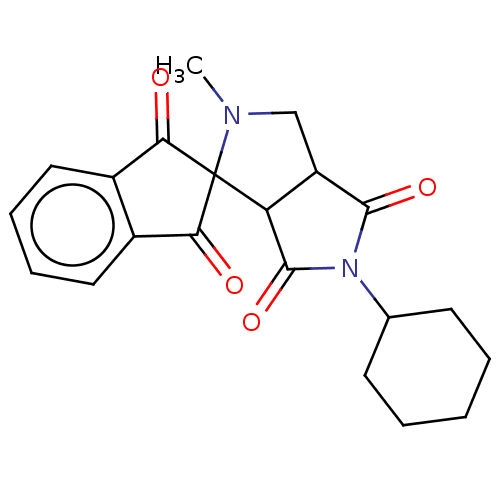 (CHEMBL4089033) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558516 (CHEMBL4797045) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242973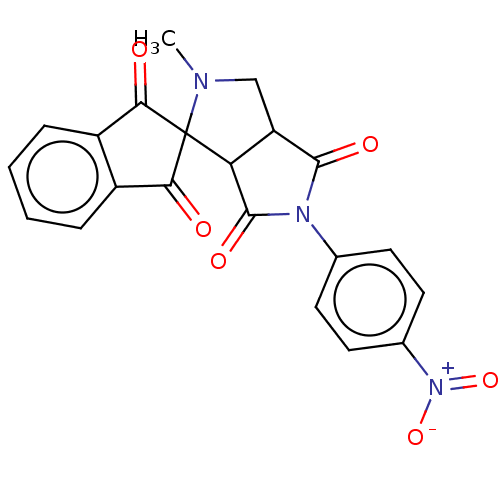 (CHEMBL4104459) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558502 (CHEMBL4760400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242979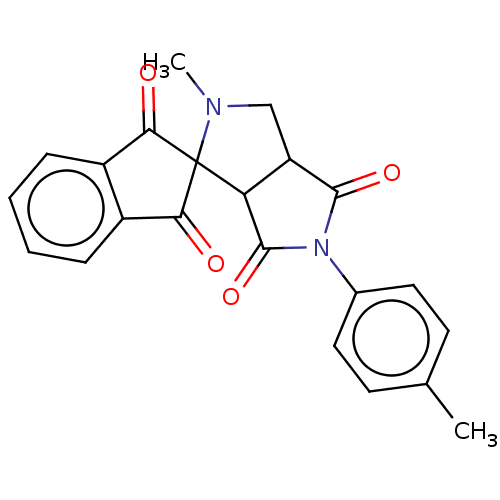 (CHEMBL4090159) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558511 (CHEMBL4754607) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242992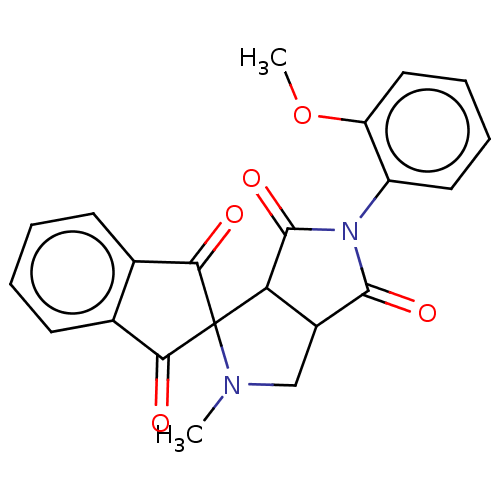 (CHEMBL4066805) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558517 (CHEMBL4800067) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558499 (CHEMBL4761620) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242993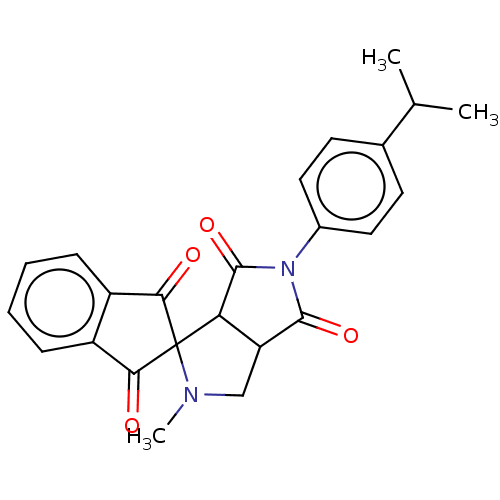 (CHEMBL4093532) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558503 (CHEMBL4754171) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558495 (CHEMBL4757955) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558494 (CHEMBL4789991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558496 (CHEMBL4797884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558515 (CHEMBL4791084) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558512 (CHEMBL4739877) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558506 (CHEMBL4788300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558507 (CHEMBL4763528) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558505 (CHEMBL4743869) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558519 (CHEMBL4744408) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558520 (CHEMBL4791351) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558509 (CHEMBL4780172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558513 (CHEMBL4799570) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558510 (CHEMBL4755928) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242981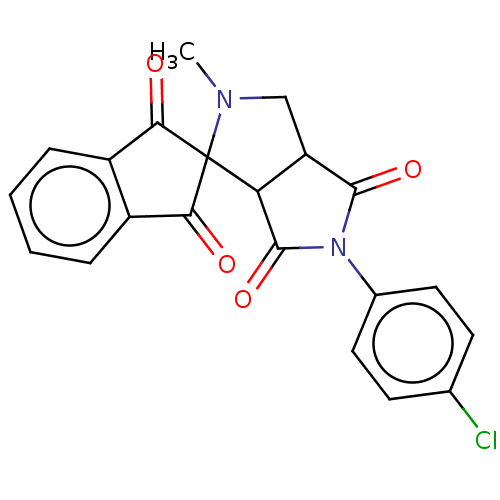 (CHEMBL4098808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558501 (CHEMBL4756742) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558518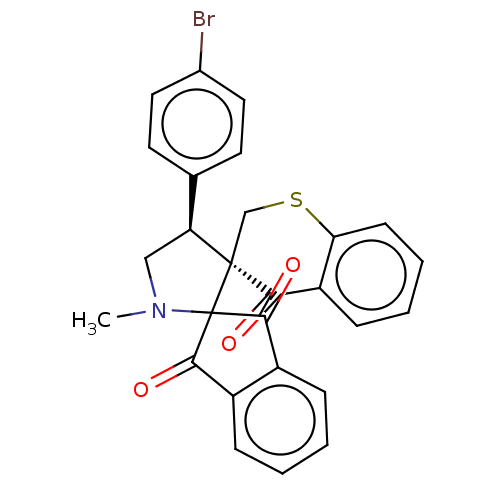 (CHEMBL4740786) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558497 (CHEMBL4748587) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558504 (CHEMBL4762214) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50558498 (CHEMBL4797575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of AChE (unknown origin) using acetylthiocholine chloride as substrate preincubated with enzyme for 15 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.044 BindingDB Entry DOI: 10.7270/Q2571GQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242969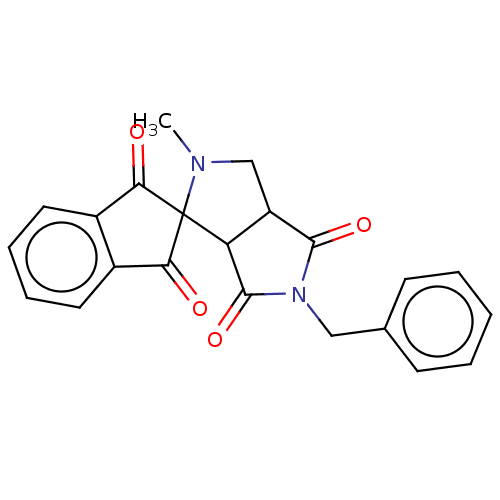 (CHEMBL4060832) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242975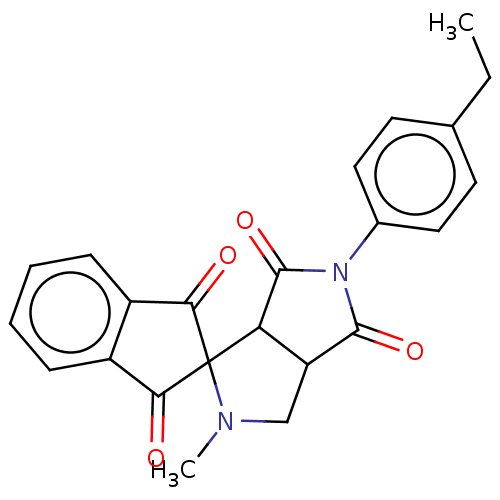 (CHEMBL4069096) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242982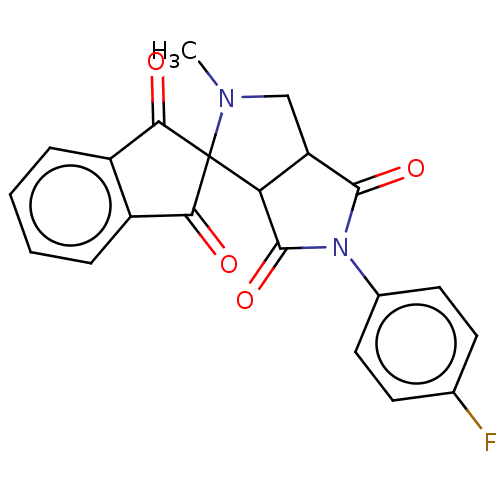 (CHEMBL4071803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242980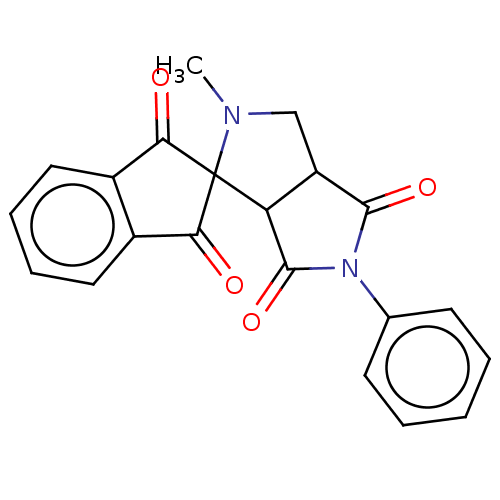 (CHEMBL4079853) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242994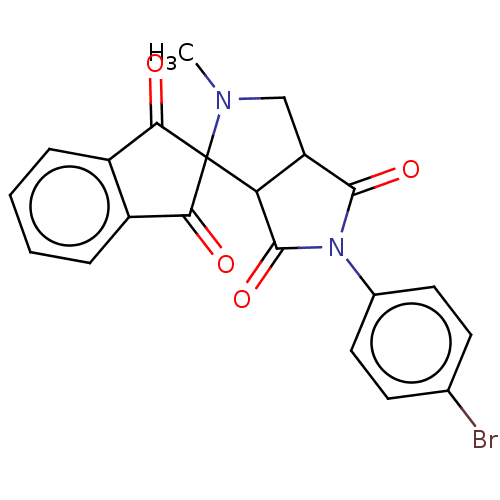 (CHEMBL4097877) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50242972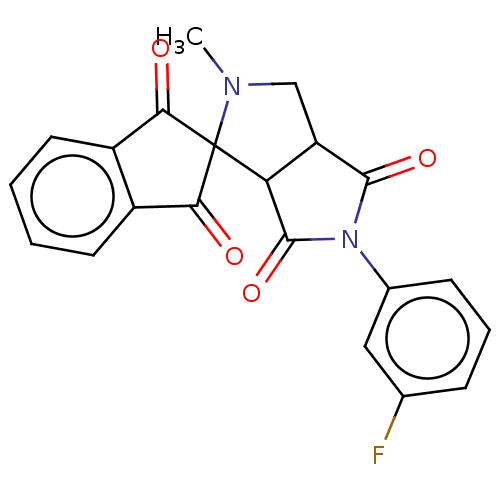 (CHEMBL4080216) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madurai Kamaraj University Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) using acetylcholine as substrate preincubated for 15 mins followed by substrate addition measured every minute by... | Bioorg Med Chem Lett 27: 3071-3075 (2017) Article DOI: 10.1016/j.bmcl.2017.05.050 BindingDB Entry DOI: 10.7270/Q2BG2RDS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||