

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM13211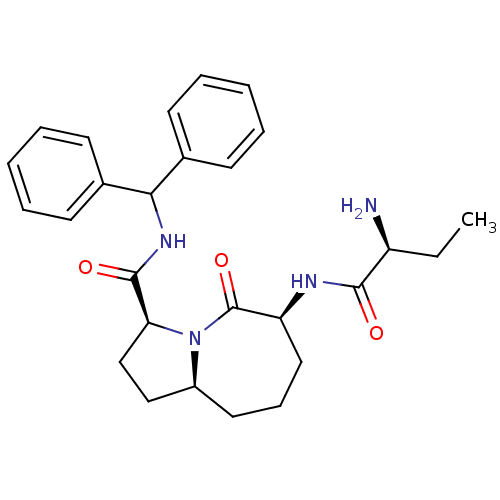 ((3S,6S,9aS)-6-[(2S)-2-aminobutanamido]-N-(diphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | -43.1 | 460 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | Bioorg Med Chem 17: 5834-56 (2009) Article DOI: 10.1016/j.bmc.2009.07.009 BindingDB Entry DOI: 10.7270/Q28W3BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM13212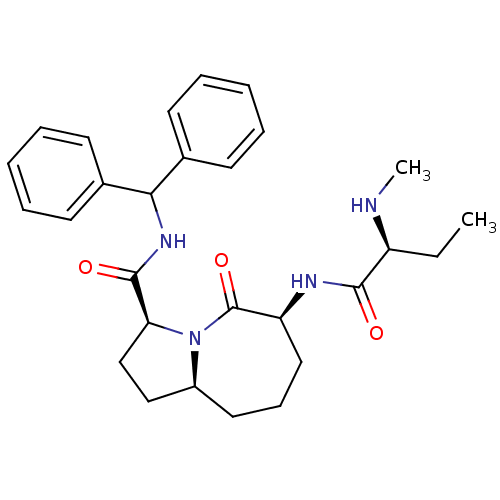 ((3S,6S,9aS)-N-(diphenylmethyl)-6-[(2S)-2-(methylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | -40.9 | 530 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Universita degli Studi di Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | Bioorg Med Chem 17: 5834-56 (2009) Article DOI: 10.1016/j.bmc.2009.07.009 BindingDB Entry DOI: 10.7270/Q28W3BNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM26205 ((3S,6S,7R,9aS)-6-[(2S)-2-aminobutanamido]-7-(2-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 220 | -37.6 | 250 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | J Mol Biol 384: 673-89 (2008) Article DOI: 10.1016/j.jmb.2008.09.064 BindingDB Entry DOI: 10.7270/Q2NG4NZ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM26203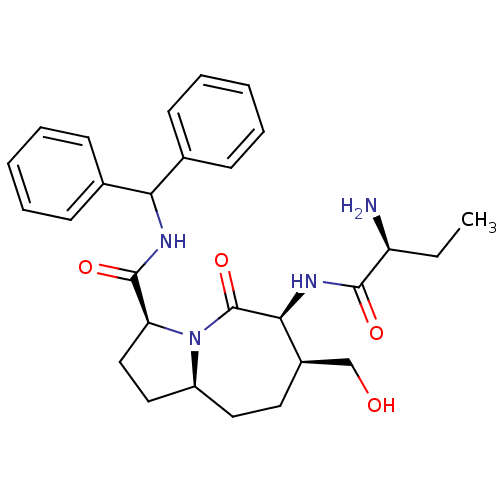 ((3S,6S,7S,9aS)-6-[(2S)-2-aminobutanamido]-N-(diphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 250 | -37.3 | 270 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | J Mol Biol 384: 673-89 (2008) Article DOI: 10.1016/j.jmb.2008.09.064 BindingDB Entry DOI: 10.7270/Q2NG4NZ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50003399 (4'-(2-Butyl-4-chloro-5-hydroxymethyl-imidazol-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM13211 ((3S,6S,9aS)-6-[(2S)-2-aminobutanamido]-N-(diphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | -36.0 | 460 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | J Mol Biol 384: 673-89 (2008) Article DOI: 10.1016/j.jmb.2008.09.064 BindingDB Entry DOI: 10.7270/Q2NG4NZ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM26204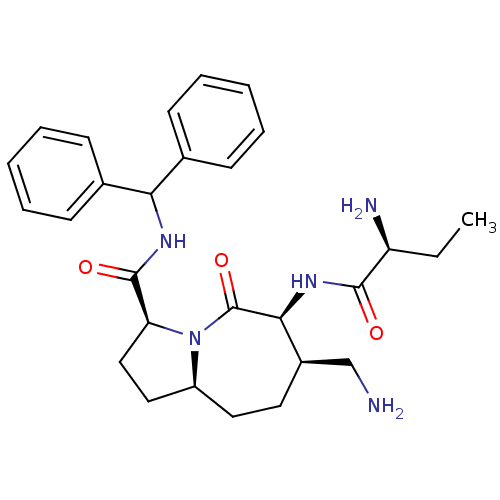 ((3S,6S,7R,9aS)-6-[(2S)-2-aminobutanamido]-7-(amino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 870 | -34.2 | 970 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of Milano | Assay Description Fluorescence polarization was measured on an Ultra plate reader (Tecan) at excitation and emission wavelengths of 485 and 530 nm, respectively. The e... | J Mol Biol 384: 673-89 (2008) Article DOI: 10.1016/j.jmb.2008.09.064 BindingDB Entry DOI: 10.7270/Q2NG4NZ8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234288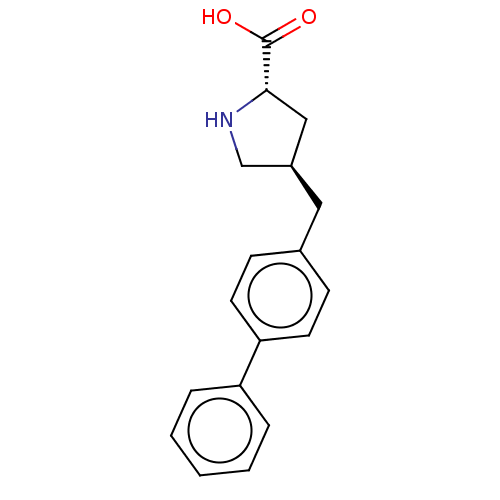 (CHEMBL4085113) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470054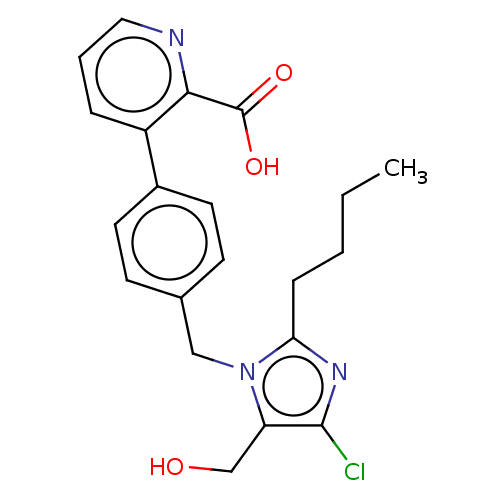 (CHEMBL129225) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470049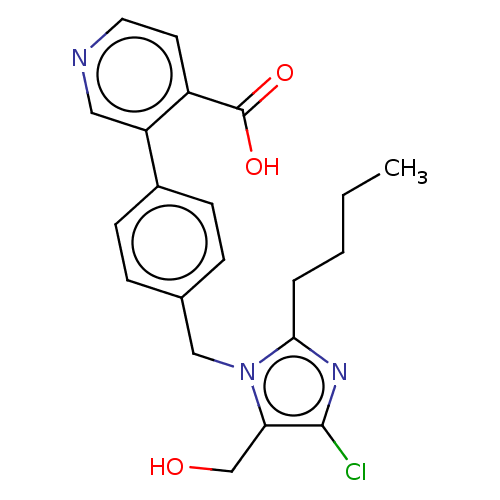 (CHEMBL340682) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470051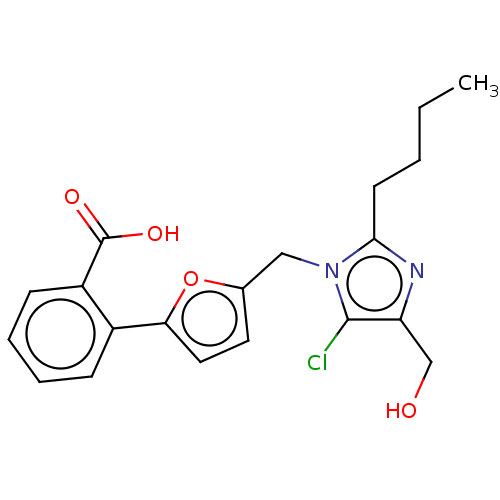 (CHEMBL339044) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470050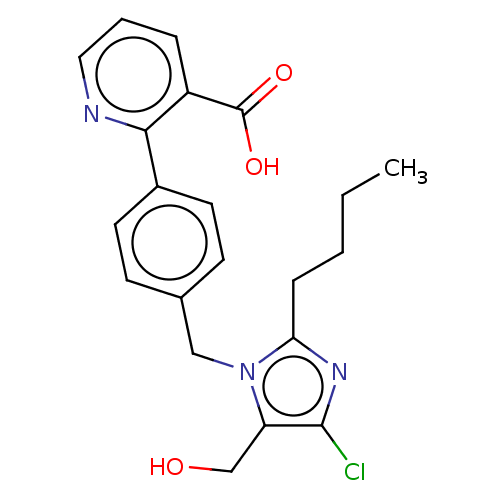 (CHEMBL129170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470052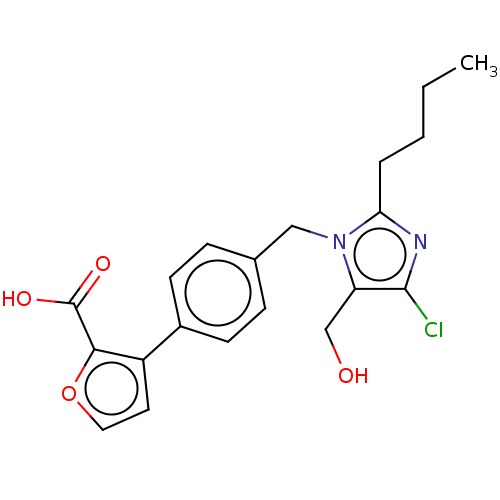 (CHEMBL127108) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470060 (CHEMBL125789) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470055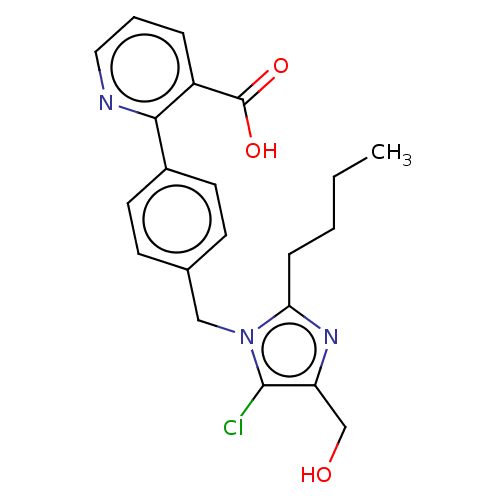 (CHEMBL125790) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470048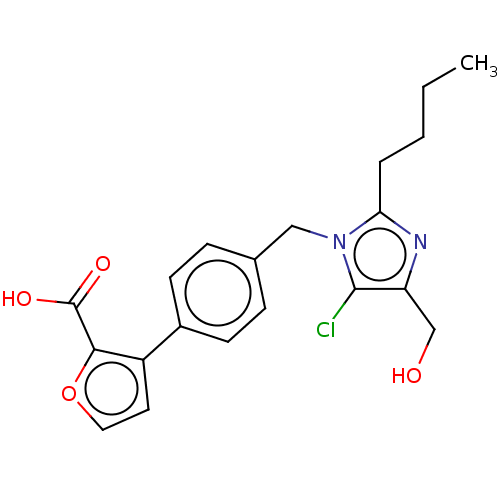 (CHEMBL128291) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470059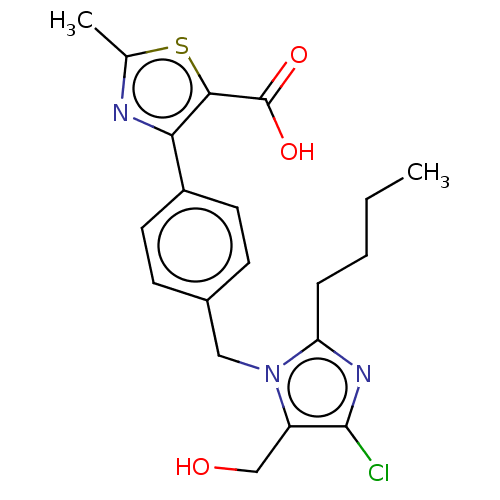 (CHEMBL338840) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470047 (CHEMBL50135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470057 (CHEMBL129336) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470058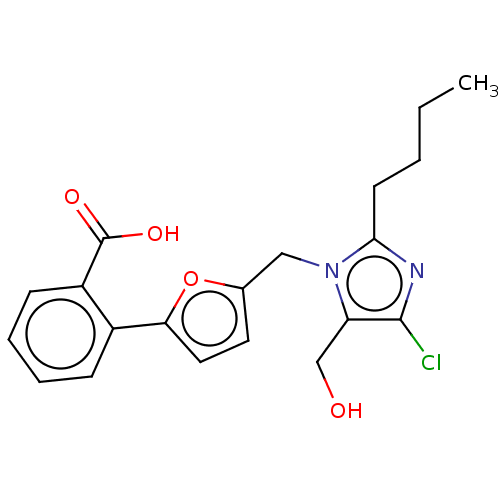 (CHEMBL129005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470053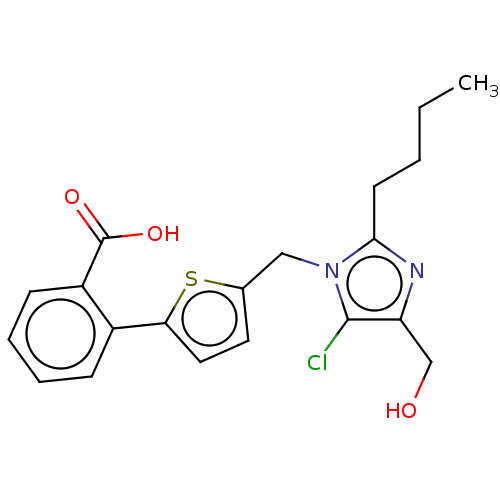 (CHEMBL125621) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor (RAT) | BDBM50470056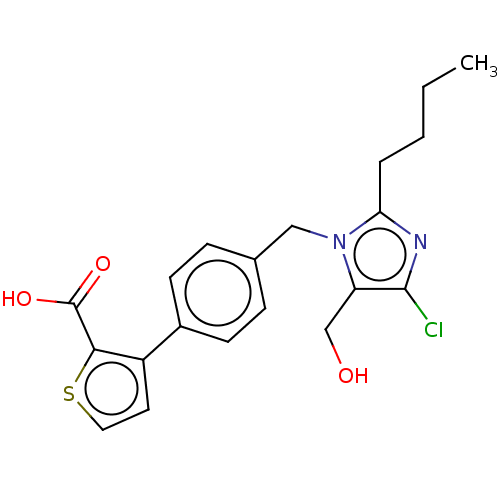 (CHEMBL129006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Lusofarmaco Curated by ChEMBL | Assay Description Tested for the binding affinity using radioligand [125I]-Sar1 Ile8-AII in the rat adrenal capsular membranes (adrenal cortex) | J Med Chem 37: 3928-38 (1994) Article DOI: 10.1021/jm00049a012 BindingDB Entry DOI: 10.7270/Q2W098NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234283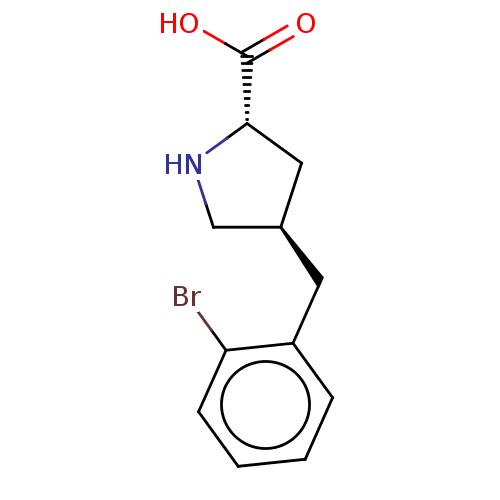 (CHEMBL4060165) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234285 (CHEMBL4083894) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234284 (CHEMBL4083473) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234291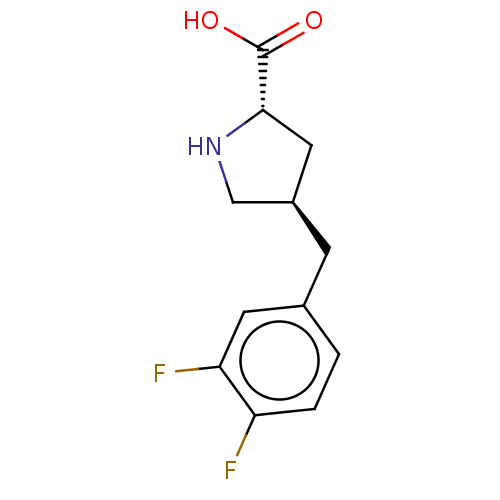 (CHEMBL4068819) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234287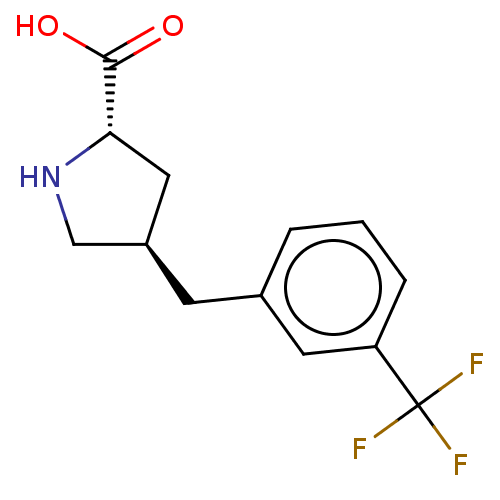 (CHEMBL4100876) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234280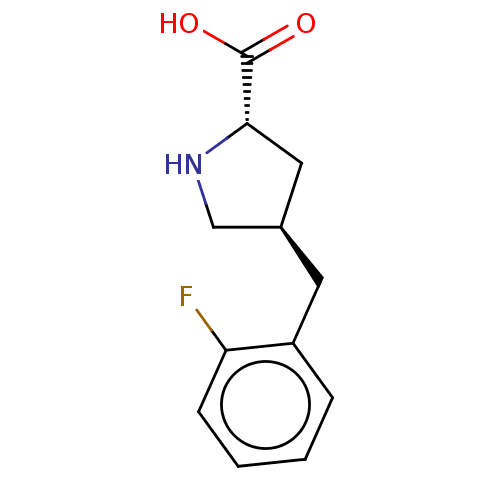 (CHEMBL4071517) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234279 (CHEMBL4078902) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234278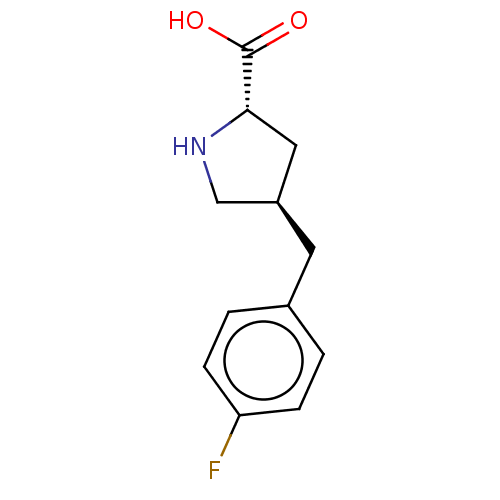 (CHEMBL4103180) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234282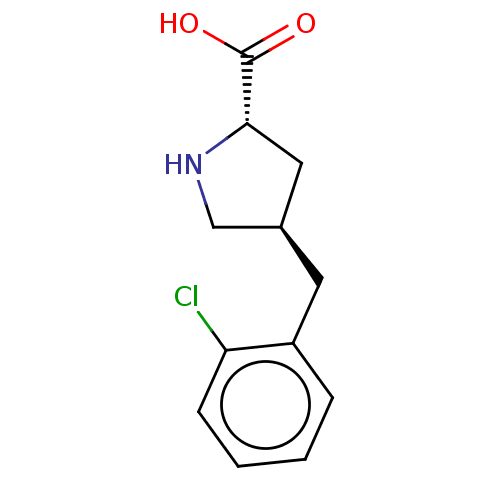 (CHEMBL4097017) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.95E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234286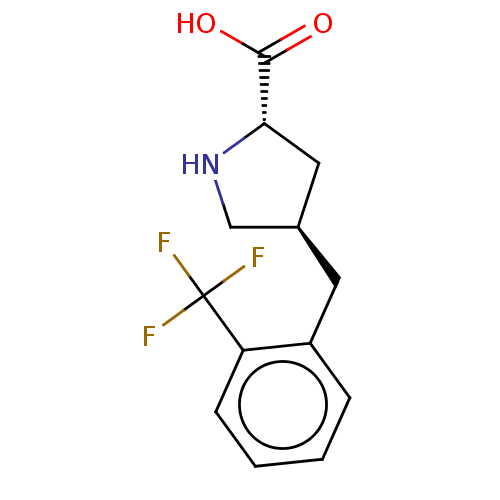 (CHEMBL4064465) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234292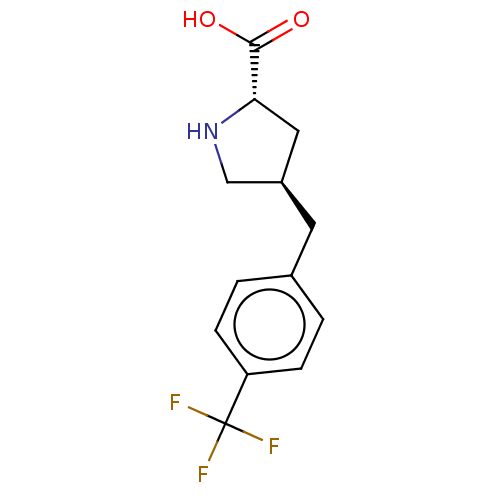 (CHEMBL4080252) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234290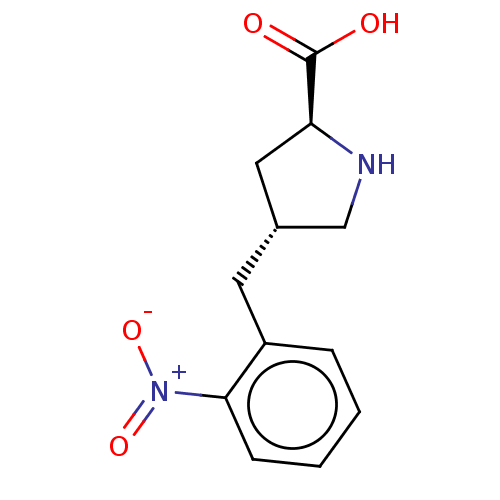 (CHEMBL4098554) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amino acid transporter (Rattus norvegicus) | BDBM50234289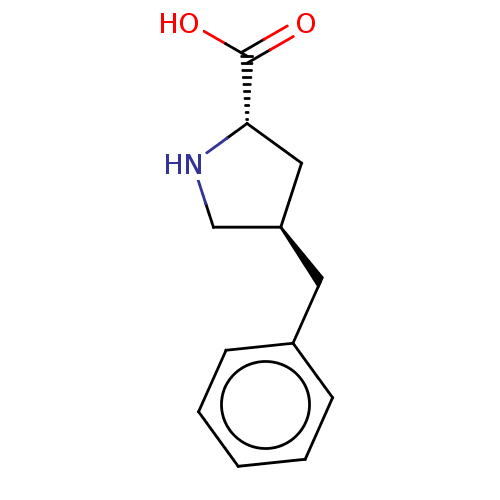 (CHEMBL4068036) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Binghamton University Curated by ChEMBL | Assay Description Inhibition of rat ASCT2 expressed in HEK293 cells assessed as inhibition of L-alanine/Na+ exchange by measuring reduction in SCN anion inward current... | Bioorg Med Chem Lett 27: 398-402 (2017) Article DOI: 10.1016/j.bmcl.2016.12.063 BindingDB Entry DOI: 10.7270/Q2HX1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538576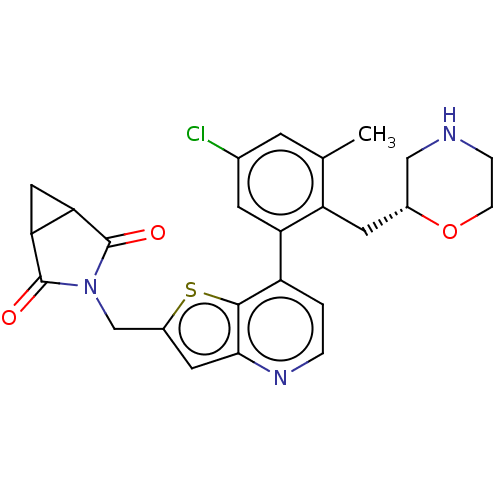 (CHEMBL4634092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538560 (CHEMBL4640002) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538571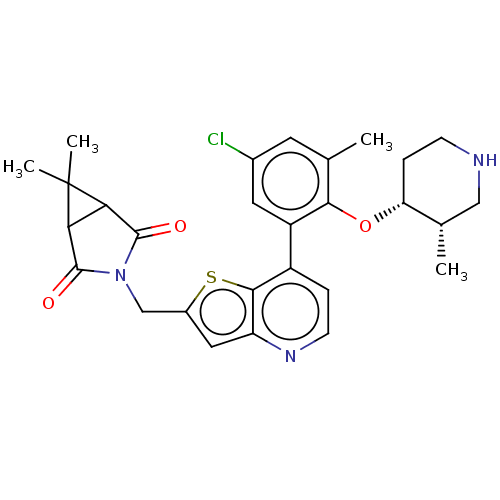 (CHEMBL4635160) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538572 (CHEMBL4649132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538575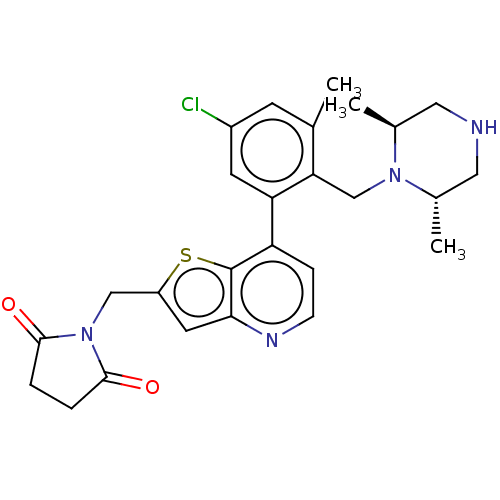 (CHEMBL4638458) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538562 (CHEMBL4643330) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538578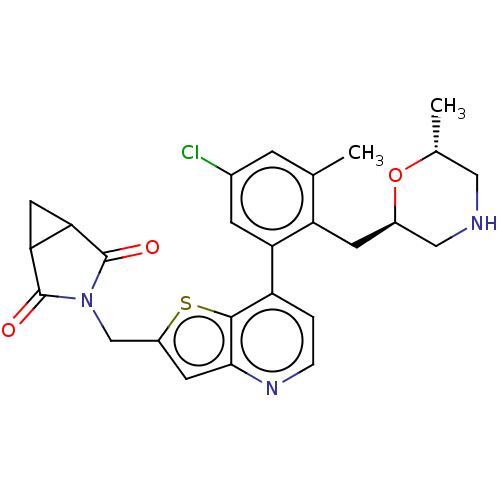 (CHEMBL4640729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538579 (CHEMBL4647217) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538582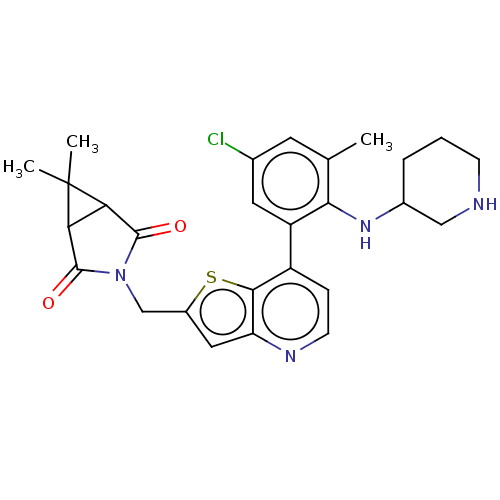 (CHEMBL4638998) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538563 (CHEMBL4648116) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538570 (CHEMBL4638573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50401475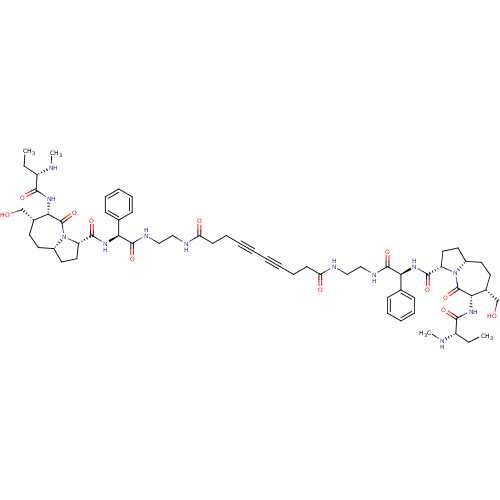 (CHEMBL2205248) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione IRCCS Istituto Nazionale dei Tumori Curated by ChEMBL | Assay Description Displacement of FITC-Smac from human recombinant His-tagged cIAP-1 BIR3 domain (245 to 357 residues) after 3 hrs by fluorescent polarization assay | Bioorg Med Chem 20: 6709-23 (2012) Article DOI: 10.1016/j.bmc.2012.09.041 BindingDB Entry DOI: 10.7270/Q2P84D2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538564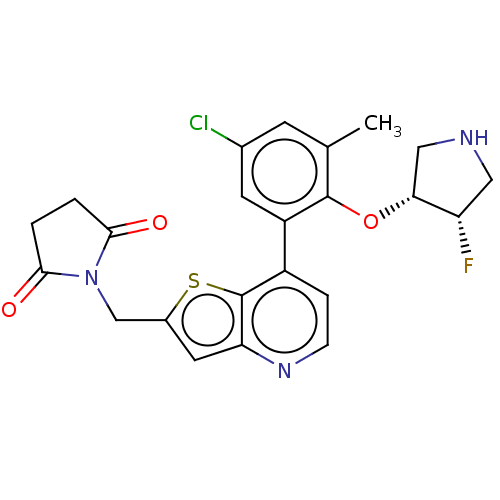 (CHEMBL4641569) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50538583 (CHEMBL4643112) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
RAPT Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant full length USP7 (unknown origin) using ubiquintin-rhodamine as substrate preincubated for 30 mins followed by substrate ad... | J Med Chem 63: 5398-5420 (2020) Article DOI: 10.1021/acs.jmedchem.0c00245 BindingDB Entry DOI: 10.7270/Q2XK8K2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50401484 (CHEMBL2204575) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fondazione IRCCS Istituto Nazionale dei Tumori Curated by ChEMBL | Assay Description Displacement of FITC-Smac from human recombinant His-tagged cIAP-1 BIR3 domain (245 to 357 residues) after 3 hrs by fluorescent polarization assay | Bioorg Med Chem 20: 6709-23 (2012) Article DOI: 10.1016/j.bmc.2012.09.041 BindingDB Entry DOI: 10.7270/Q2P84D2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 462 total ) | Next | Last >> |