

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418177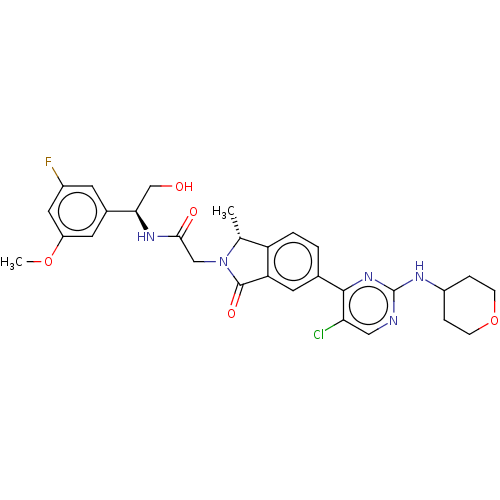 (2-[(1R)-5-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50573875 (CHEMBL4869086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417999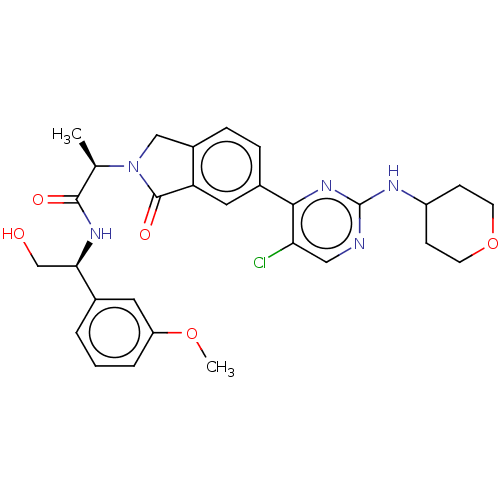 (US10457669, Example 675 | US11001575, Example 675) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418270 ((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50573874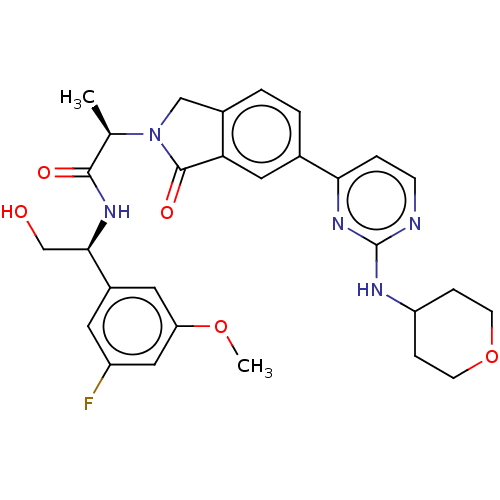 (CHEMBL4873851) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418019 (US10457669, Example 698 | US11001575, Example 698) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418018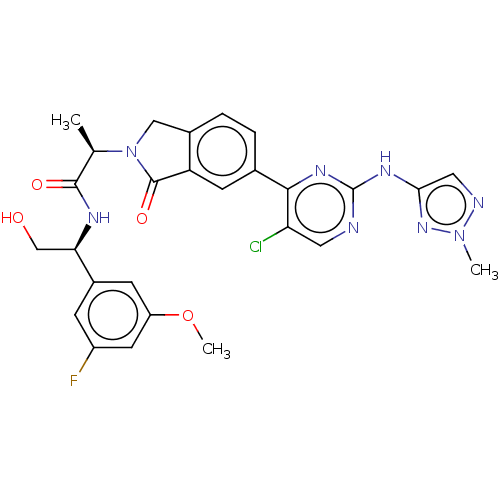 (US10457669, Example 697 | US11001575, Example 697) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456355 (CHEMBL4207147 | US11001575, Example 554) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1/3 (Homo sapiens (Human)) | BDBM50456348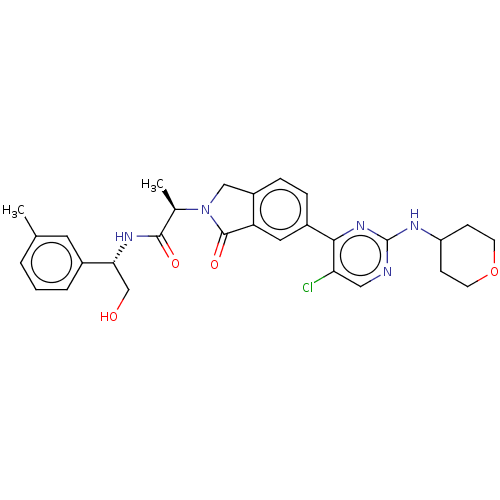 (CHEMBL4212211 | US11001575, Example 683) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ERK1/2 phosphorylation in human A375 cells harboring BRAF V600E mutant after 4 hrs by ELISA | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456356 (CHEMBL4209691 | US11001575, Example 674) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50573872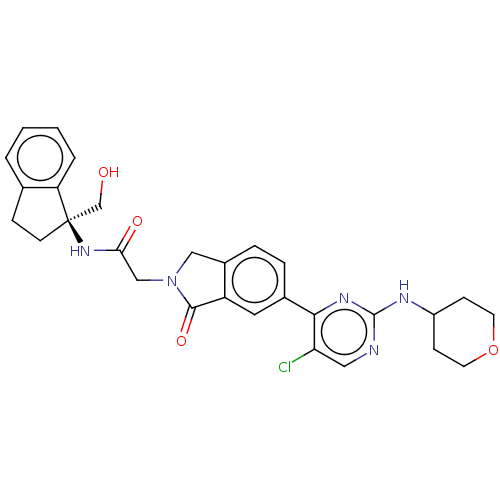 (CHEMBL4868536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418007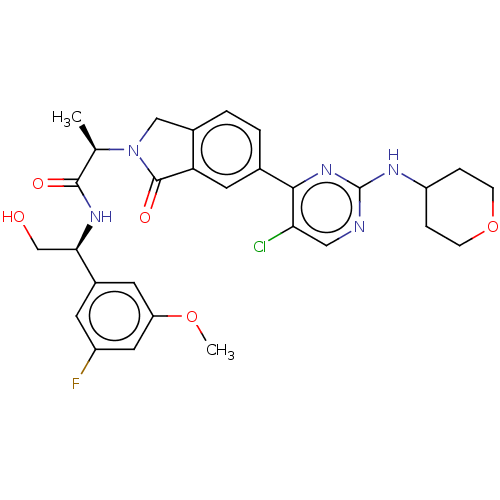 (US10457669, Example 1083 | US10457669, Example 685...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418315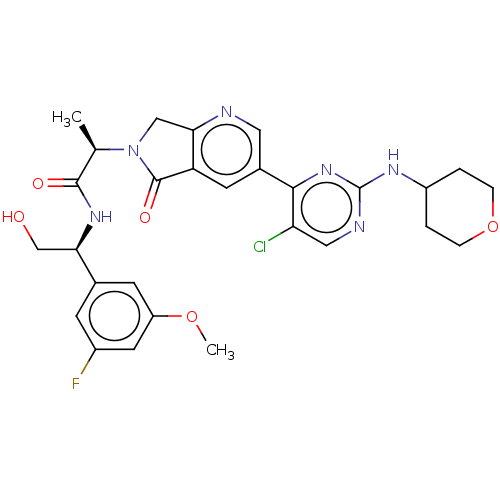 ((2R)-2-(3-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093001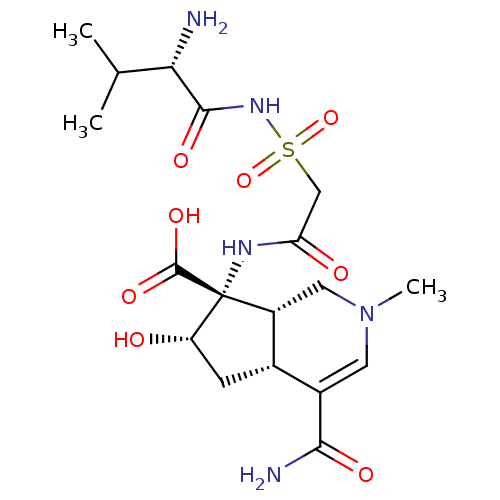 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456348 (CHEMBL4212211 | US11001575, Example 683) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456348 (CHEMBL4212211 | US11001575, Example 683) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418226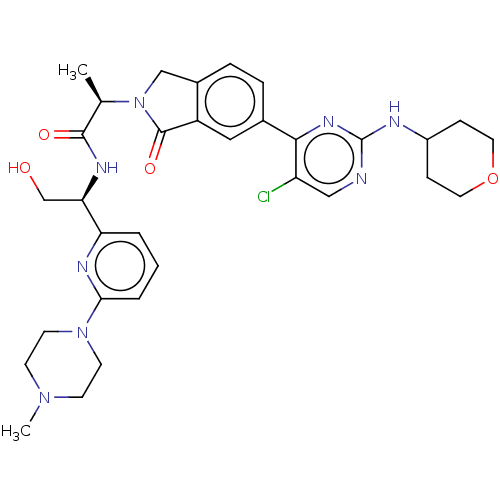 ((2R)-2-(6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50573873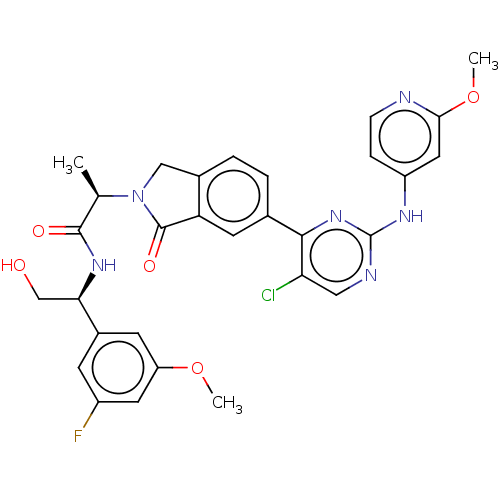 (CHEMBL4870176) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1/3 (Homo sapiens (Human)) | BDBM50456348 (CHEMBL4212211 | US11001575, Example 683) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ERK1/2 in human A375 cells harboring BRAF V600E mutant assessed as suppression of RSK phosphorylation after 4 hrs by MSD assay | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456353 (CHEMBL4210682 | US11001575, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456347 (CHEMBL4207117 | US11001575, Example 80) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093003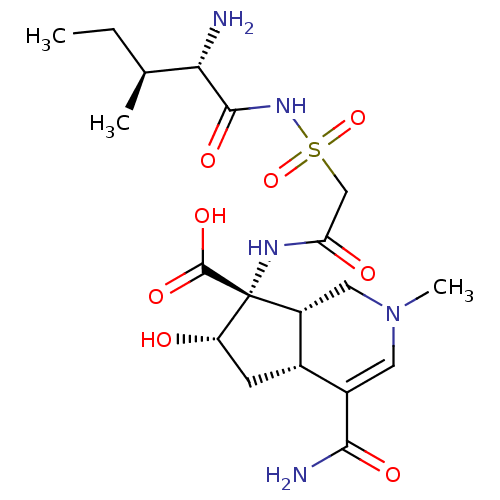 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456351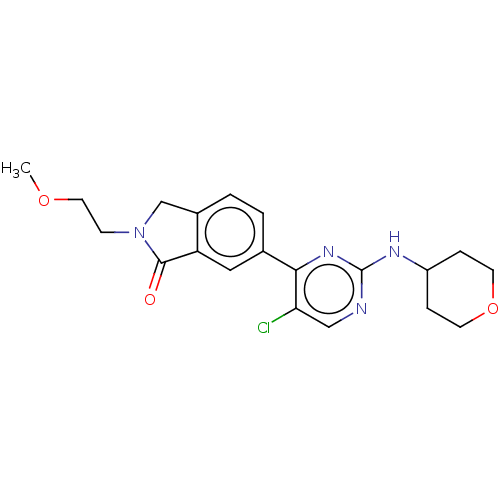 (CHEMBL4217740 | US11001575, Example 103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456361 (CHEMBL4215376 | US11001575, Example 141) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417431 (6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin-4- yl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456362 (CHEMBL4206693 | US11001575, Example 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456346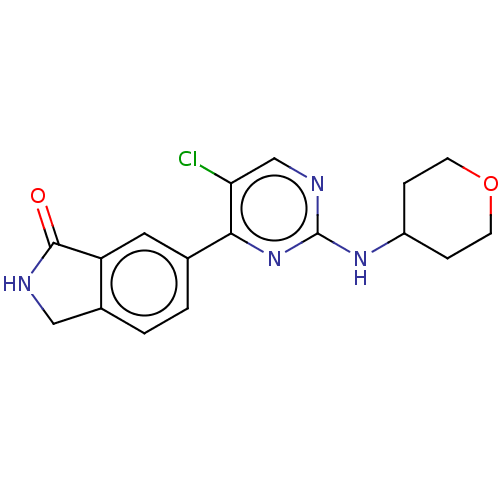 (CHEMBL4204392 | US11001575, Example 128) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM418281 ((2R)-2-[6-(5-chloro-2-{[(2S)-1- hydroxypropan-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417432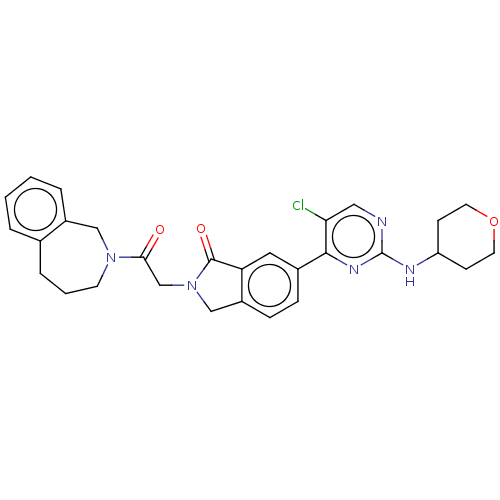 (6-{5-chloro-2-[(oxan-4- yl)amino]pyrimidin-4- yl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50093005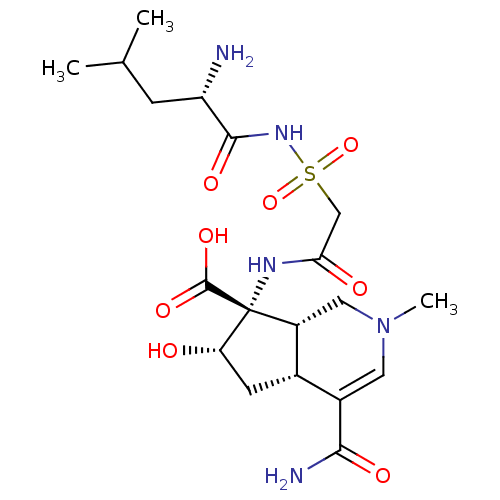 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093004 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456352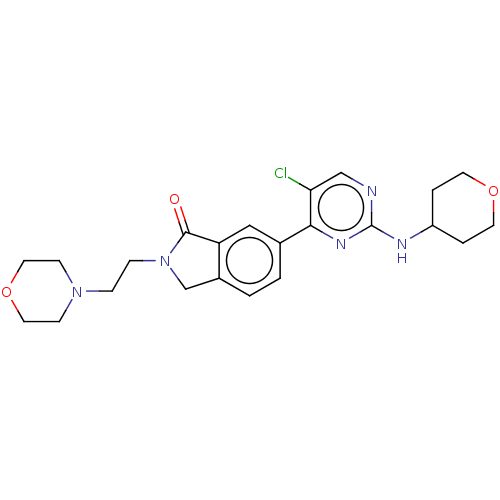 (CHEMBL4213926 | US11001575, Example 104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456350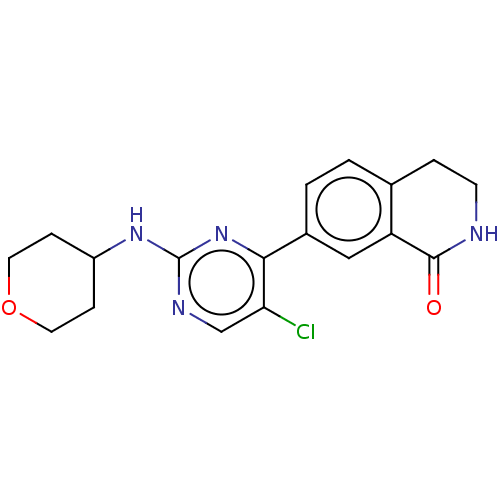 (CHEMBL4207587 | US11001575, Example 132) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093002 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM417517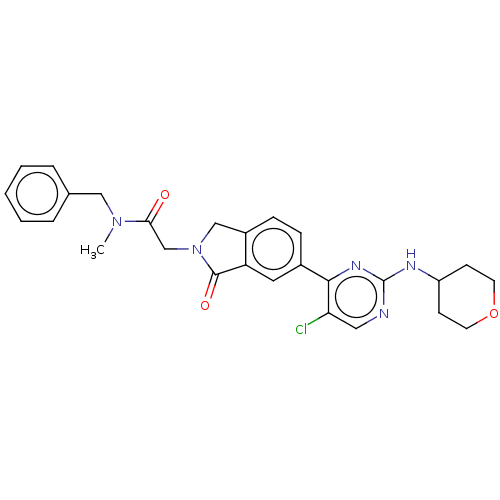 (N-benzyl-2-(6-{5-chloro- 2-[(oxan-4-yl)amino] pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456358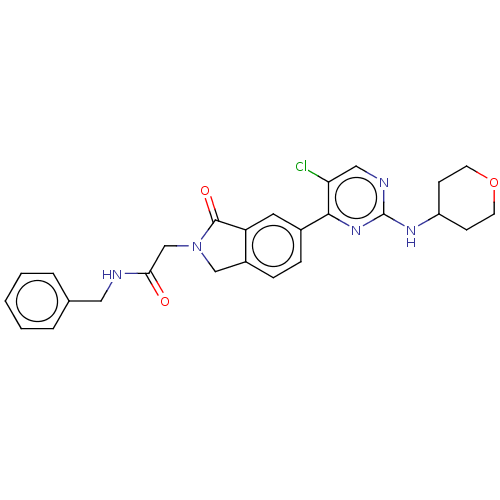 (CHEMBL4213466 | US11001575, Example 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length human N-terminal MAHHHHHH tagged-ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate incubated for 30... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00905 BindingDB Entry DOI: 10.7270/Q2BP06M5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456358 (CHEMBL4213466 | US11001575, Example 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093001 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093001 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456359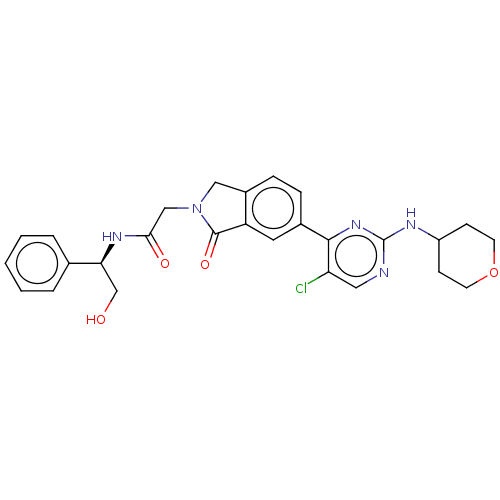 (CHEMBL4206737 | US11001575, Example 409) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093004 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456354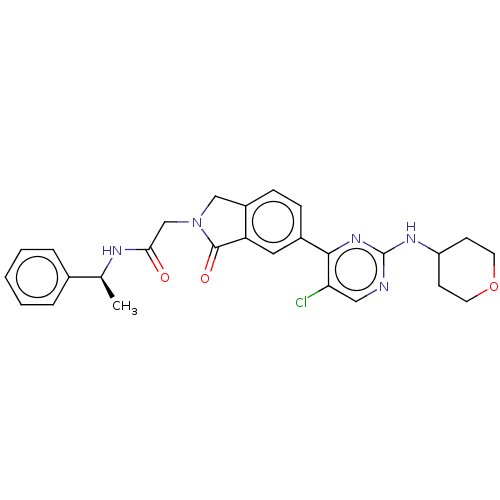 (CHEMBL4202882 | US11001575, Example 410) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093005 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093002 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093005 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 1 (Homo sapiens (Human)) | BDBM50456357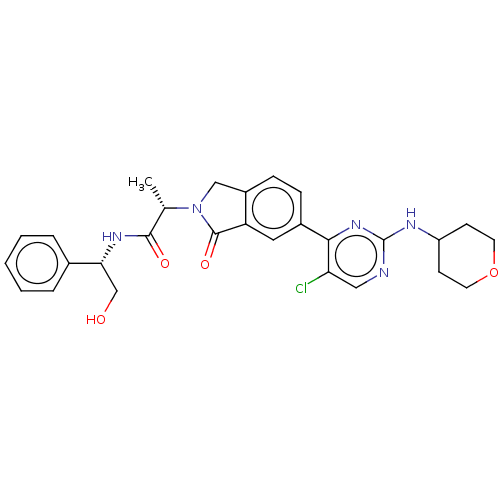 (CHEMBL4213211) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of full length human N-terminal MAHHHHHH-tagged ERK2 expressed in Escherichia coli BL21 (DE3) using ATF2-GFP as substrate after 30 mins by... | J Med Chem 61: 4978-4992 (2018) Article DOI: 10.1021/acs.jmedchem.8b00421 BindingDB Entry DOI: 10.7270/Q2HT2RZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093006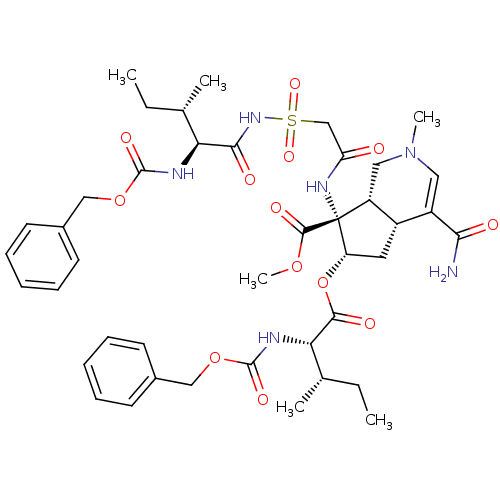 ((4aR,6S,7R,7aS)-6-((2S,3S)-2-Benzyloxycarbonylamin...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50593237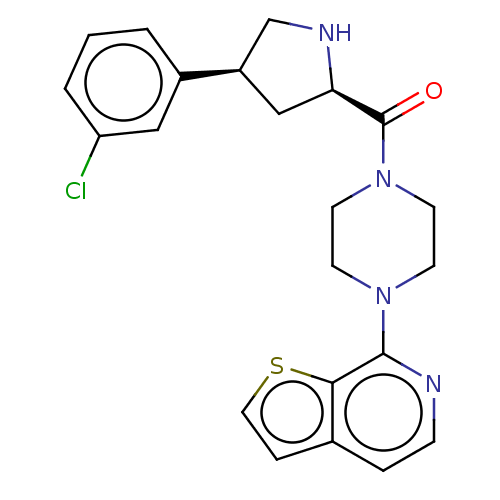 (CHEMBL5199411) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00272 BindingDB Entry DOI: 10.7270/Q2N01BHR | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |