 Found 46 hits with Last Name = 'pattison' and Initial = 'c'
Found 46 hits with Last Name = 'pattison' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185404

((+/-)-(4aS,6R,8aS)-6-(4-chloro-benzenesulfonyl)-1-...)Show SMILES Fc1ccc(F)c(c1)[C@]1(CC[C@H]2[C@H](CNS(=O)(=O)N2C2CC2)C1)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H23ClF2N2O4S2/c23-15-1-6-18(7-2-15)32(28,29)22(19-11-16(24)3-8-20(19)25)10-9-21-14(12-22)13-26-33(30,31)27(21)17-4-5-17/h1-3,6-8,11,14,17,21,26H,4-5,9-10,12-13H2/t14-,21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185410

((4aS,6S,8aR)-6-(4-chloro-benzenesulfonyl)-6-(2,5-d...)Show SMILES CCC1C[C@@H]2C[C@](CC[C@@H]2NS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H24ClF2NO4S2/c1-2-17-11-14-13-22(10-9-21(14)26-32(17,29)30,19-12-16(24)5-8-20(19)25)31(27,28)18-6-3-15(23)4-7-18/h3-8,12,14,17,21,26H,2,9-11,13H2,1H3/t14-,17?,21+,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185411
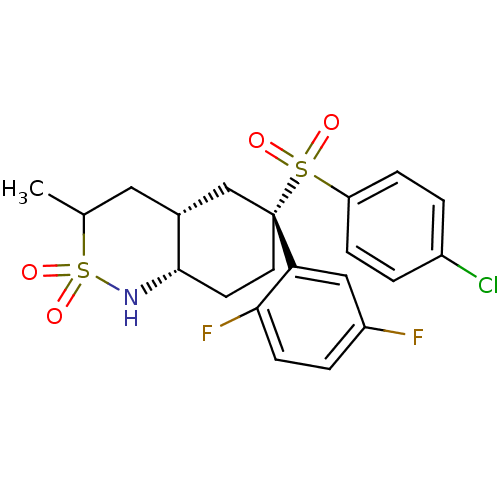
((4aS,6S,8aR)-6-(4-chloro-benzenesulfonyl)-6-(2,5-d...)Show SMILES CC1C[C@@H]2C[C@](CC[C@@H]2NS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H22ClF2NO4S2/c1-13-10-14-12-21(9-8-20(14)25-31(13,28)29,18-11-16(23)4-7-19(18)24)30(26,27)17-5-2-15(22)3-6-17/h2-7,11,13-14,20,25H,8-10,12H2,1H3/t13?,14-,20+,21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185407

((+/-)-(4aS,6R,8aS)-6-(4-chloro-benzenesulfonyl)-3-...)Show SMILES Fc1ccc(F)c(c1)[C@]1(CC[C@@H]2NS(=O)(=O)N(C[C@@H]2C1)C1CC1)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H23ClF2N2O4S2/c23-15-1-6-18(7-2-15)32(28,29)22(19-11-16(24)3-8-20(19)25)10-9-21-14(12-22)13-27(17-4-5-17)33(30,31)26-21/h1-3,6-8,11,14,17,21,26H,4-5,9-10,12-13H2/t14-,21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185414

((4aS,6S,8aR)-6-(4-chloro-benzenesulfonyl)-6-(2,5-d...)Show SMILES CCCC1C[C@@H]2C[C@](CC[C@@H]2NS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H26ClF2NO4S2/c1-2-3-19-12-15-14-23(11-10-22(15)27-33(19,30)31,20-13-17(25)6-9-21(20)26)32(28,29)18-7-4-16(24)5-8-18/h4-9,13,15,19,22,27H,2-3,10-12,14H2,1H3/t15-,19?,22+,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185405

((+/-)-(4aS,6R,8aS)-6-(4-chloro-benzenesulfonyl)-3-...)Show SMILES Fc1ccc(F)c(c1)[C@]1(CC[C@@H]2NS(=O)(=O)N(C[C@@H]2C1)C1CCC1)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H25ClF2N2O4S2/c24-16-4-7-19(8-5-16)33(29,30)23(20-12-17(25)6-9-21(20)26)11-10-22-15(13-23)14-28(18-2-1-3-18)34(31,32)27-22/h4-9,12,15,18,22,27H,1-3,10-11,13-14H2/t15-,22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]haloperidol binding to dopamine receptor in rat striatal homogenate |
J Med Chem 28: 1811-7 (1985)
BindingDB Entry DOI: 10.7270/Q2KW5J7H |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50334150
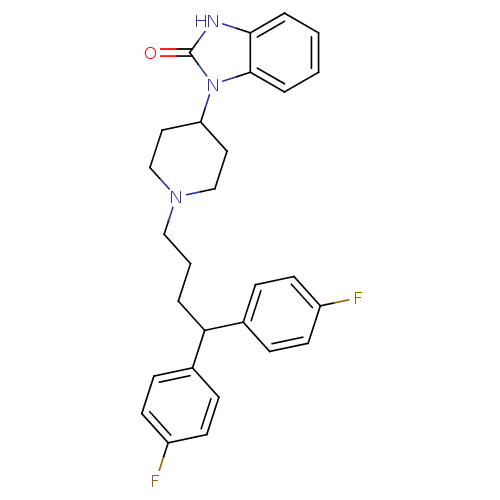
(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. |
J Med Chem 28: 606-12 (1985)
BindingDB Entry DOI: 10.7270/Q2TB193Z |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50334150

(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. |
J Med Chem 28: 606-12 (1985)
BindingDB Entry DOI: 10.7270/Q2TB193Z |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185413
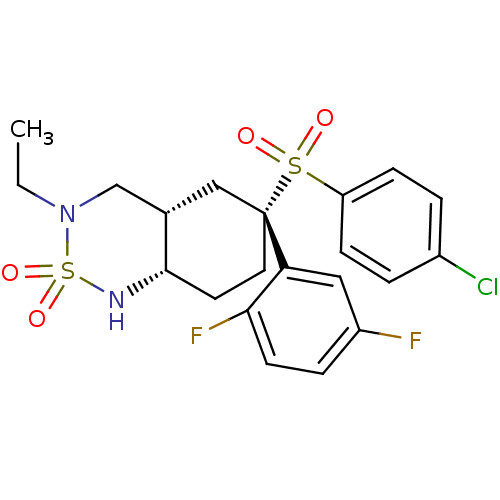
((+/-)-(4aS,6R,8aS)-6-(4-chloro-benzenesulfonyl)-6-...)Show SMILES CCN1C[C@@H]2C[C@](CC[C@@H]2NS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClF2N2O4S2/c1-2-26-13-14-12-21(10-9-20(14)25-32(26,29)30,18-11-16(23)5-8-19(18)24)31(27,28)17-6-3-15(22)4-7-17/h3-8,11,14,20,25H,2,9-10,12-13H2,1H3/t14-,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185408

((+/-)-(4aS,6R,8aS)-6-(4-chloro-benzenesulfonyl)-6-...)Show SMILES CC(C)N1C[C@@H]2C[C@](CC[C@@H]2NS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H25ClF2N2O4S2/c1-14(2)27-13-15-12-22(10-9-21(15)26-33(27,30)31,19-11-17(24)5-8-20(19)25)32(28,29)18-6-3-16(23)4-7-18/h3-8,11,14-15,21,26H,9-10,12-13H2,1-2H3/t15-,21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225369

(CHEMBL295712)Show SMILES Fc1ccc(cc1)N(CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C28H30F2N4O/c29-22-7-11-24(12-8-22)33(25-13-9-23(30)10-14-25)18-4-17-32-19-15-28(16-20-32)27(35)31-21-34(28)26-5-2-1-3-6-26/h1-3,5-14H,4,15-21H2,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. |
J Med Chem 28: 606-12 (1985)
BindingDB Entry DOI: 10.7270/Q2TB193Z |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185406

((4aS,6S,8aR)-6-(4-chloro-benzenesulfonyl)-6-(2,5-d...)Show SMILES CCC1C[C@@H]2C[C@](CC[C@@H]2CS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H25ClF2O4S2/c1-2-19-11-16-13-23(10-9-15(16)14-31(19,27)28,21-12-18(25)5-8-22(21)26)32(29,30)20-6-3-17(24)4-7-20/h3-8,12,15-16,19H,2,9-11,13-14H2,1H3/t15-,16-,19?,23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185412
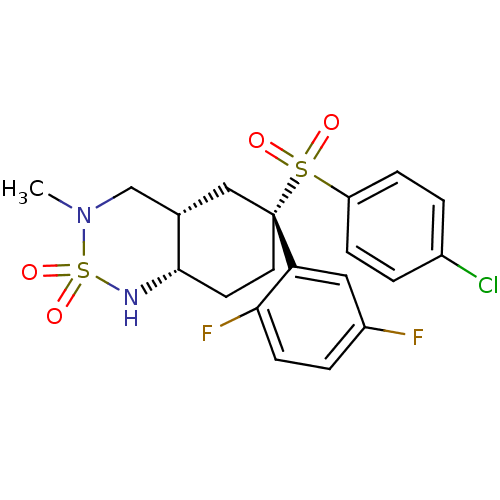
((+/-)-(4aS,6R,8aS)-6-(4-chloro-benzenesulfonyl)-6-...)Show SMILES CN1C[C@@H]2C[C@](CC[C@@H]2NS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C20H21ClF2N2O4S2/c1-25-12-13-11-20(9-8-19(13)24-31(25,28)29,17-10-15(22)4-7-18(17)23)30(26,27)16-5-2-14(21)3-6-16/h2-7,10,13,19,24H,8-9,11-12H2,1H3/t13-,19-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185409

((+/-)-(4aS,6R,8aS)-6-(4-chloro-benzenesulfonyl)-6-...)Show SMILES CCCN1C[C@@H]2C[C@](CC[C@@H]2NS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H25ClF2N2O4S2/c1-2-11-27-14-15-13-22(10-9-21(15)26-33(27,30)31,19-12-17(24)5-8-20(19)25)32(28,29)18-6-3-16(23)4-7-18/h3-8,12,15,21,26H,2,9-11,13-14H2,1H3/t15-,21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185403

((+/-)-(4aS,6R,8aS)-3-tert-butyl-6-(4-chloro-benzen...)Show SMILES CC(C)(C)N1C[C@@H]2C[C@](CC[C@@H]2NS1(=O)=O)(c1cc(F)ccc1F)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C23H27ClF2N2O4S2/c1-22(2,3)28-14-15-13-23(11-10-21(15)27-34(28,31)32,19-12-17(25)6-9-20(19)26)33(29,30)18-7-4-16(24)5-8-18/h4-9,12,15,21,27H,10-11,13-14H2,1-3H3/t15-,21-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50002338
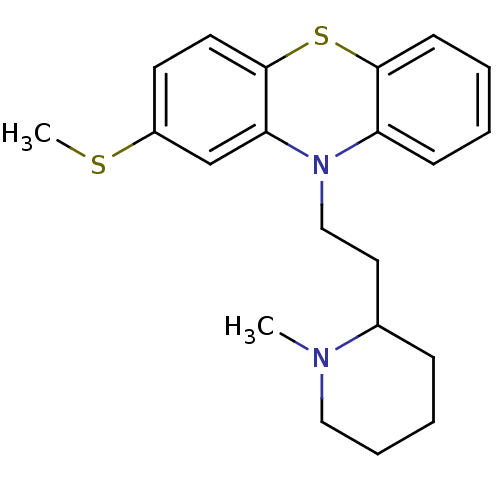
((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
Gamma-secretase subunit APH-1A/Gamma-secretase subunit APH-1B/Gamma-secretase subunit PEN-2/Nicastrin/Presenilin-1/Presenilin-2
(Homo sapiens (Human)) | BDBM50185402
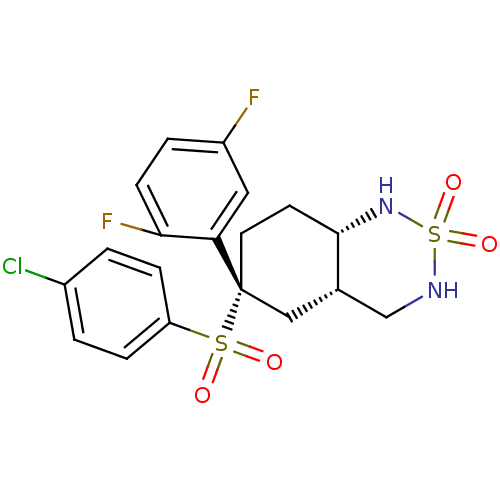
((+/-)-(4aS,6R,8aS)-6-(4-chloro-benzenesulfonyl)-6-...)Show SMILES Fc1ccc(F)c(c1)[C@]1(CC[C@@H]2NS(=O)(=O)NC[C@@H]2C1)S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H19ClF2N2O4S2/c20-13-1-4-15(5-2-13)29(25,26)19(16-9-14(21)3-6-17(16)22)8-7-18-12(10-19)11-23-30(27,28)24-18/h1-6,9,12,18,23-24H,7-8,10-11H2/t12-,18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of gamma secretase |
Bioorg Med Chem Lett 16: 3073-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.083
BindingDB Entry DOI: 10.7270/Q2DR2V3C |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50225510
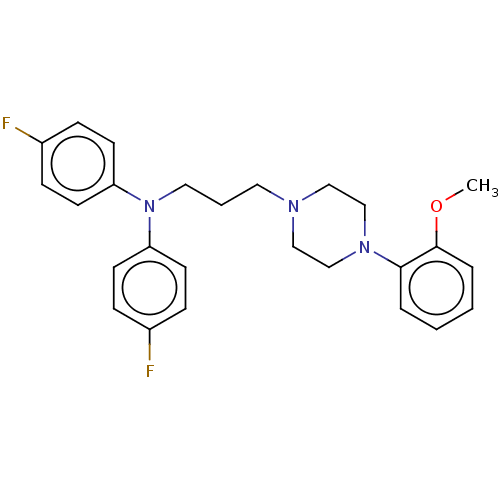
(CHEMBL156395)Show SMILES COc1ccccc1N1CCN(CCCN(c2ccc(F)cc2)c2ccc(F)cc2)CC1 Show InChI InChI=1S/C26H29F2N3O/c1-32-26-6-3-2-5-25(26)30-19-17-29(18-20-30)15-4-16-31(23-11-7-21(27)8-12-23)24-13-9-22(28)10-14-24/h2-3,5-14H,4,15-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. |
J Med Chem 28: 606-12 (1985)
BindingDB Entry DOI: 10.7270/Q2TB193Z |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50002338

((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
Affinity for displacement of [3H]-WB-4101 labeled Dopamine receptor D1 |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of [3H]WB-4101 binding to alpha-1 adrenergic receptor of rat frontal cortex |
J Med Chem 28: 1811-7 (1985)
BindingDB Entry DOI: 10.7270/Q2KW5J7H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of [3H]QNB binding to Muscarinic acetylcholine receptor |
J Med Chem 28: 1811-7 (1985)
BindingDB Entry DOI: 10.7270/Q2KW5J7H |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of [3H]QNB binding to Muscarinic acetylcholine receptor |
J Med Chem 28: 1811-7 (1985)
BindingDB Entry DOI: 10.7270/Q2KW5J7H |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [3H]haloperidol binding to dopamine receptor in rat striatal homogenate |
J Med Chem 28: 1811-7 (1985)
BindingDB Entry DOI: 10.7270/Q2KW5J7H |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]haloperidol binding to dopamine receptors in rat striatal membranes. |
J Med Chem 28: 606-12 (1985)
BindingDB Entry DOI: 10.7270/Q2TB193Z |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50225369

(CHEMBL295712)Show SMILES Fc1ccc(cc1)N(CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C28H30F2N4O/c29-22-7-11-24(12-8-22)33(25-13-9-23(30)10-14-25)18-4-17-32-19-15-28(16-20-32)27(35)31-21-34(28)26-5-2-1-3-6-26/h1-3,5-14H,4,15-21H2,(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of [3H]QNB binding to Muscarinic acetylcholine receptor |
J Med Chem 28: 1811-7 (1985)
BindingDB Entry DOI: 10.7270/Q2KW5J7H |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50225369

(CHEMBL295712)Show SMILES Fc1ccc(cc1)N(CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1)c1ccc(F)cc1 Show InChI InChI=1S/C28H30F2N4O/c29-22-7-11-24(12-8-22)33(25-13-9-23(30)10-14-25)18-4-17-32-19-15-28(16-20-32)27(35)31-21-34(28)26-5-2-1-3-6-26/h1-3,5-14H,4,15-21H2,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of rabbit plasma renin. |
J Med Chem 28: 1811-7 (1985)
BindingDB Entry DOI: 10.7270/Q2KW5J7H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019909
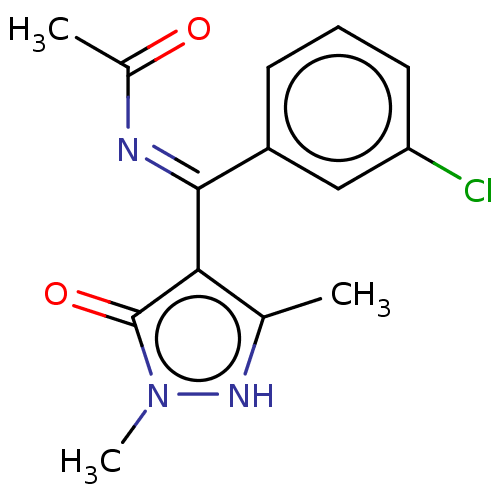
(CHEMBL3144632 | N-[(3-Chloro-phenyl)-(5-hydroxy-1,...)Show SMILES CC(=O)\N=C(\c1c(C)[nH]n(C)c1=O)c1cccc(Cl)c1 Show InChI InChI=1S/C14H14ClN3O2/c1-8-12(14(20)18(3)17-8)13(16-9(2)19)10-5-4-6-11(15)7-10/h4-7,17H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019916
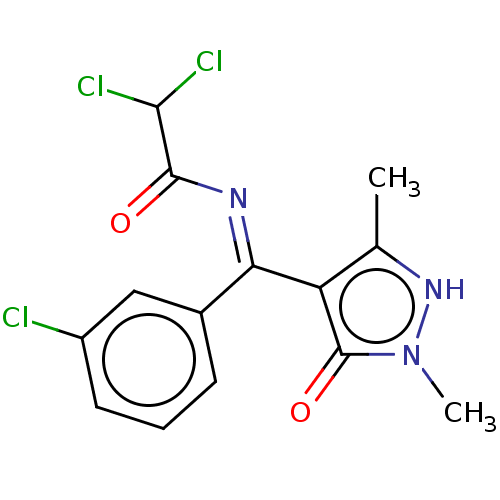
(2,2-Dichloro-N-[(3-chloro-phenyl)-(5-hydroxy-1,3-d...)Show SMILES Cc1[nH]n(C)c(=O)c1\C(=N\C(=O)C(Cl)Cl)c1cccc(Cl)c1 Show InChI InChI=1S/C14H12Cl3N3O2/c1-7-10(14(22)20(2)19-7)11(18-13(21)12(16)17)8-4-3-5-9(15)6-8/h3-6,12,19H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50002338

((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
Affinity for displacement of [3H]-clonidine labeled Dopamine receptor D1 |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of [3H]WB-4101 binding to alpha-1 adrenergic receptor of rat frontal cortex |
J Med Chem 28: 1811-7 (1985)
BindingDB Entry DOI: 10.7270/Q2KW5J7H |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019908
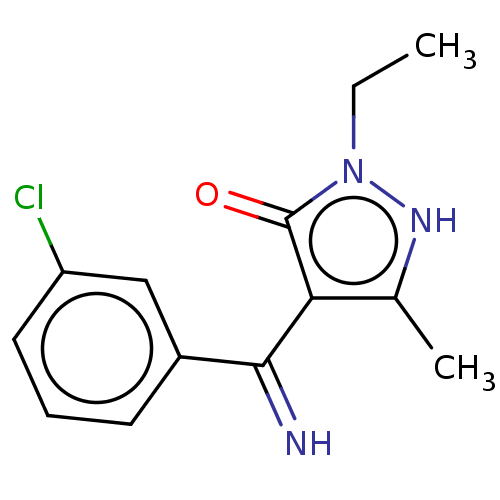
(4-[(3-Chloro-phenyl)-imino-methyl]-2-ethyl-5-methy...)Show InChI InChI=1S/C13H14ClN3O/c1-3-17-13(18)11(8(2)16-17)12(15)9-5-4-6-10(14)7-9/h4-7,15-16H,3H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019910
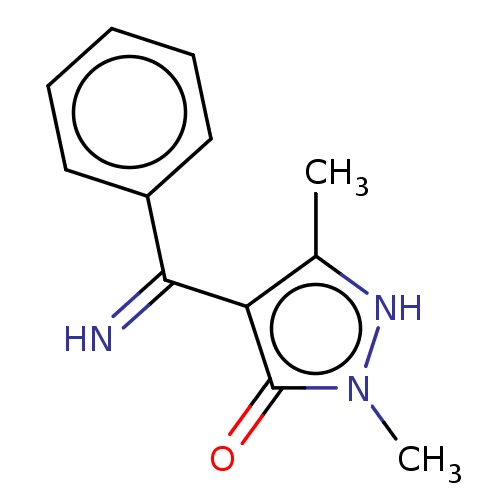
(4-(Imino-phenyl-methyl)-2,5-dimethyl-2H-pyrazol-3-...)Show InChI InChI=1S/C12H13N3O/c1-8-10(12(16)15(2)14-8)11(13)9-6-4-3-5-7-9/h3-7,13-14H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50019913
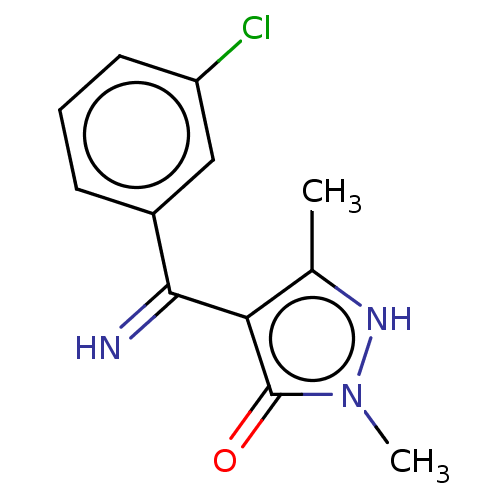
(4-[(3-Chloro-phenyl)-imino-methyl]-2,5-dimethyl-2H...)Show InChI InChI=1S/C12H12ClN3O/c1-7-10(12(17)16(2)15-7)11(14)8-4-3-5-9(13)6-8/h3-6,14-15H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
Affinity for displacement of [3H]-SCH-23,390 labeled Dopamine receptor D1 |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50019913

(4-[(3-Chloro-phenyl)-imino-methyl]-2,5-dimethyl-2H...)Show InChI InChI=1S/C12H12ClN3O/c1-7-10(12(17)16(2)15-7)11(14)8-4-3-5-9(13)6-8/h3-6,14-15H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
Affinity for displacement of [3H]-WB-4101 labeled Dopamine receptor D1 |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019911
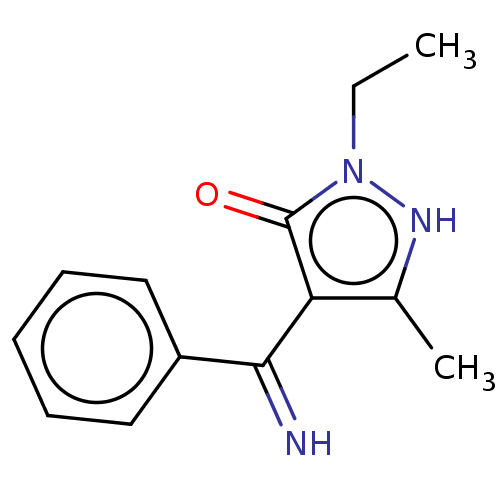
(2-Ethyl-4-(imino-phenyl-methyl)-5-methyl-2H-pyrazo...)Show InChI InChI=1S/C13H15N3O/c1-3-16-13(17)11(9(2)15-16)12(14)10-7-5-4-6-8-10/h4-8,14-15H,3H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019912
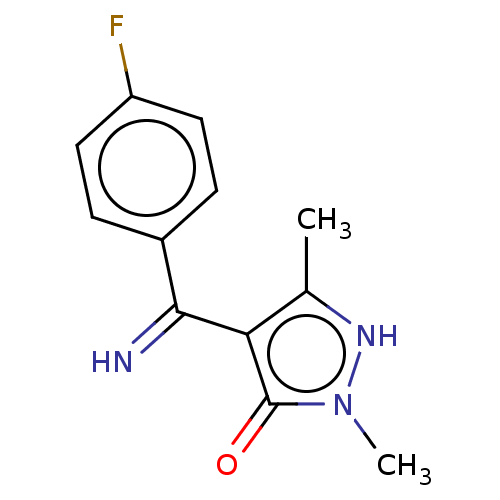
(4-[(4-Fluoro-phenyl)-imino-methyl]-2,5-dimethyl-2H...)Show InChI InChI=1S/C12H12FN3O/c1-7-10(12(17)16(2)15-7)11(14)8-3-5-9(13)6-4-8/h3-6,14-15H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM50019913

(4-[(3-Chloro-phenyl)-imino-methyl]-2,5-dimethyl-2H...)Show InChI InChI=1S/C12H12ClN3O/c1-7-10(12(17)16(2)15-7)11(14)8-4-3-5-9(13)6-8/h3-6,14-15H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
Affinity for displacement of [3H]-clonidine labeled Dopamine receptor D1 |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019914
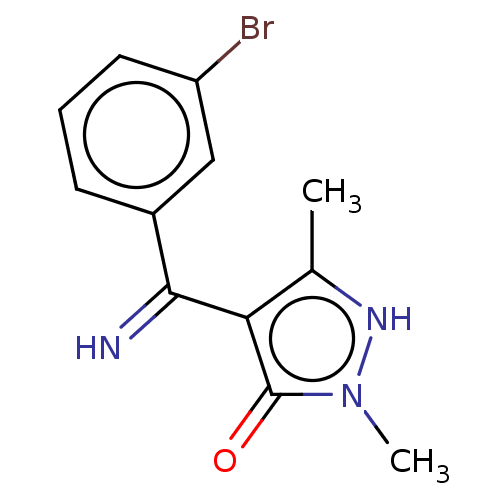
(4-[(3-Bromo-phenyl)-imino-methyl]-2,5-dimethyl-2H-...)Show InChI InChI=1S/C12H12BrN3O/c1-7-10(12(17)16(2)15-7)11(14)8-4-3-5-9(13)6-8/h3-6,14-15H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019915
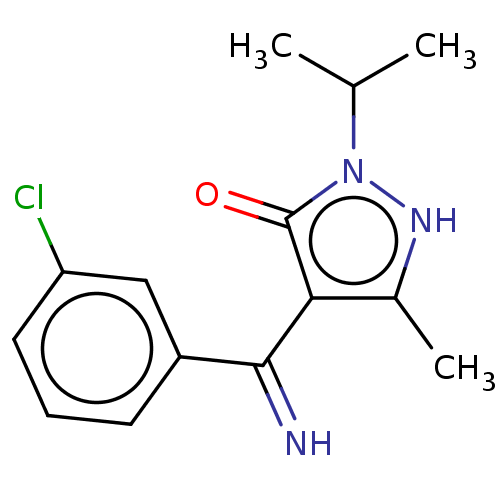
(4-[(3-Chloro-phenyl)-imino-methyl]-2-isopropyl-5-m...)Show InChI InChI=1S/C14H16ClN3O/c1-8(2)18-14(19)12(9(3)17-18)13(16)10-5-4-6-11(15)7-10/h4-8,16-17H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019913

(4-[(3-Chloro-phenyl)-imino-methyl]-2,5-dimethyl-2H...)Show InChI InChI=1S/C12H12ClN3O/c1-7-10(12(17)16(2)15-7)11(14)8-4-3-5-9(13)6-8/h3-6,14-15H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019918
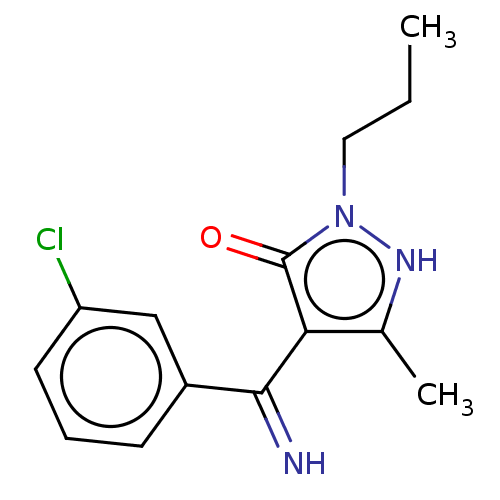
(4-[(3-Chloro-phenyl)-imino-methyl]-5-methyl-2-prop...)Show InChI InChI=1S/C14H16ClN3O/c1-3-7-18-14(19)12(9(2)17-18)13(16)10-5-4-6-11(15)8-10/h4-6,8,16-17H,3,7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019917
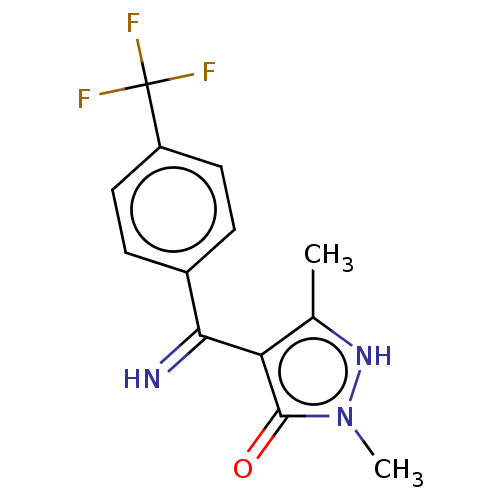
(4-[Imino-(4-trifluoromethyl-phenyl)-methyl]-2,5-di...)Show InChI InChI=1S/C13H12F3N3O/c1-7-10(12(20)19(2)18-7)11(17)8-3-5-9(6-4-8)13(14,15)16/h3-6,17-18H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019920
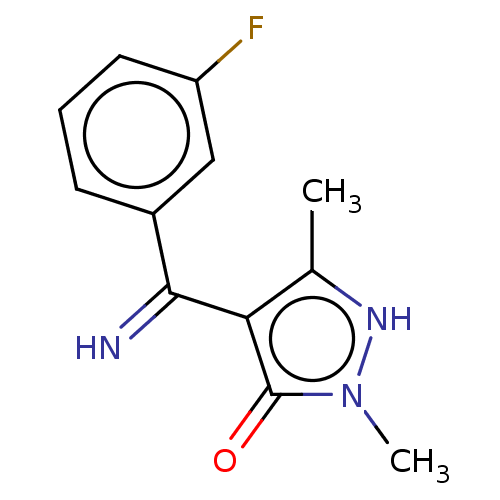
(4-[(3-Fluoro-phenyl)-imino-methyl]-2,5-dimethyl-2H...)Show InChI InChI=1S/C12H12FN3O/c1-7-10(12(17)16(2)15-7)11(14)8-4-3-5-9(13)6-8/h3-6,14-15H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50019919
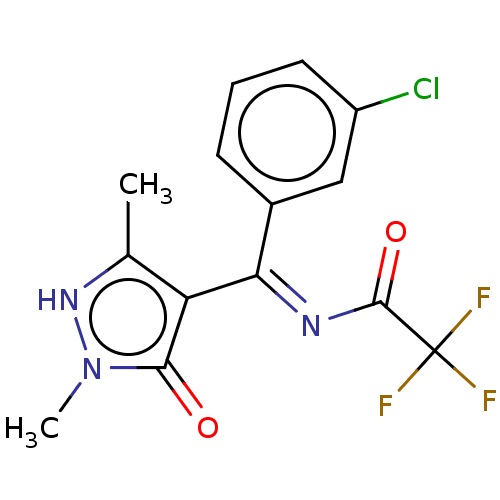
(CHEMBL3144848 | N-[(3-Chloro-phenyl)-(5-hydroxy-1,...)Show SMILES Cc1[nH]n(C)c(=O)c1\C(=N\C(=O)C(F)(F)F)c1cccc(Cl)c1 Show InChI InChI=1S/C14H11ClF3N3O2/c1-7-10(12(22)21(2)20-7)11(19-13(23)14(16,17)18)8-4-3-5-9(15)6-8/h3-6,20H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against Dopamine receptor D2 by displacement of [3H]-haloperidol from rat striatal membranes. |
J Med Chem 30: 1807-12 (1987)
BindingDB Entry DOI: 10.7270/Q29K497N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data