

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382512 (CHEMBL2022238) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382508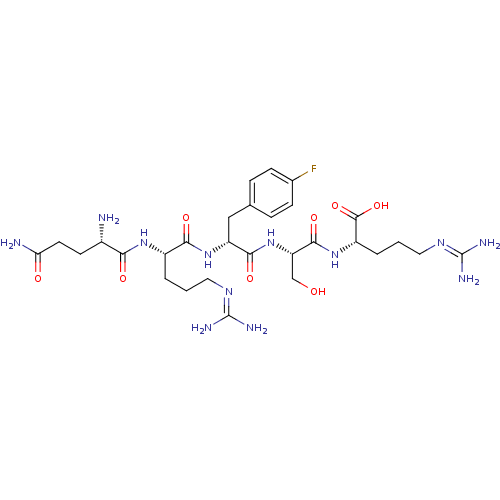 (CHEMBL2022234) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382929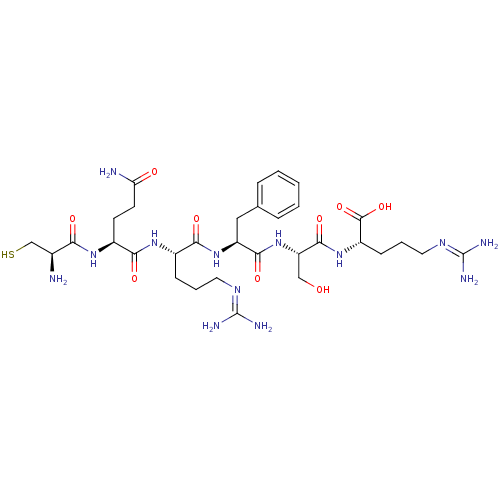 (CHEMBL2029399) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human aminopeptidase N | ACS Med Chem Lett 3: 20-24 (2012) Article DOI: 10.1021/ml200182v BindingDB Entry DOI: 10.7270/Q2P84CZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382929 (CHEMBL2029399) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human neprilysin | ACS Med Chem Lett 3: 20-24 (2012) Article DOI: 10.1021/ml200182v BindingDB Entry DOI: 10.7270/Q2P84CZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382926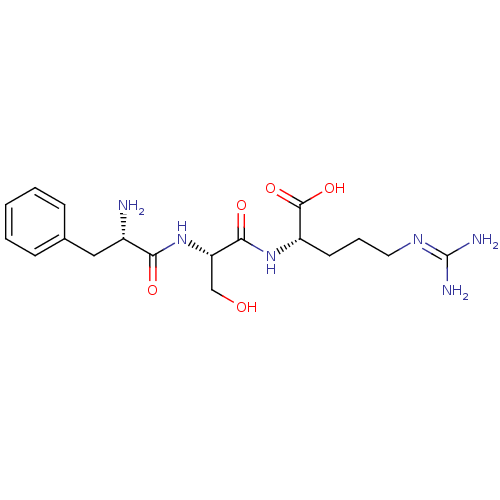 (CHEMBL2029393) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human neprilysin | ACS Med Chem Lett 3: 20-24 (2012) Article DOI: 10.1021/ml200182v BindingDB Entry DOI: 10.7270/Q2P84CZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382499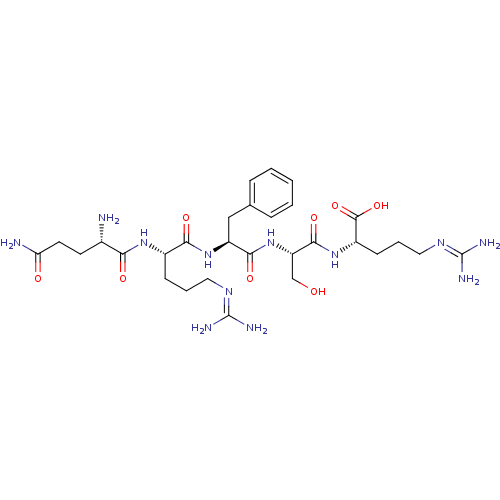 (CHEMBL2022225) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using Ala-Mca as substrate incubated for 10 mins prior to substrate addition measured for 40 mins by... | ACS Med Chem Lett 3: 20-24 (2012) Article DOI: 10.1021/ml200182v BindingDB Entry DOI: 10.7270/Q2P84CZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382499 (CHEMBL2022225) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382499 (CHEMBL2022225) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382515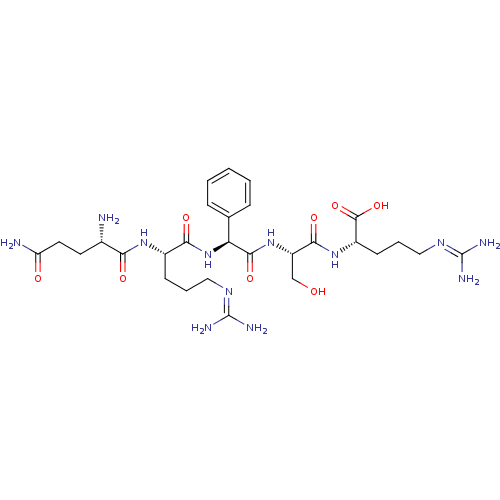 (CHEMBL2024267) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382520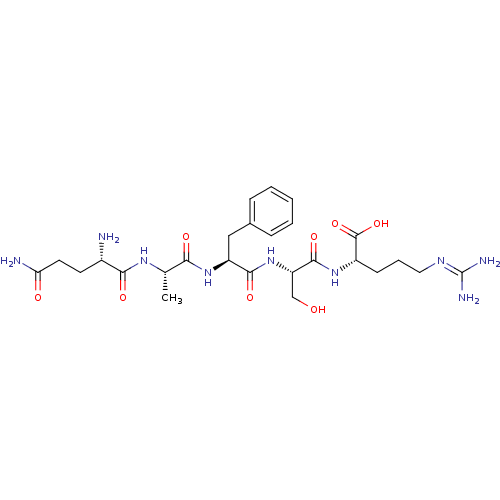 (CHEMBL2022227) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382507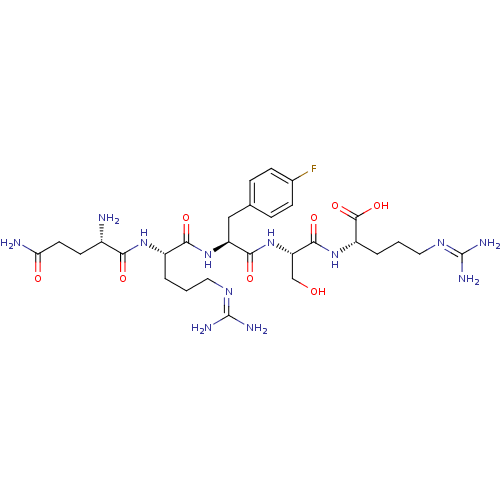 (CHEMBL2021931) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131538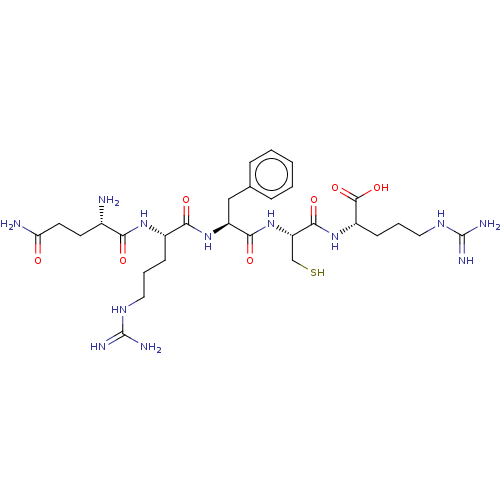 (CHEMBL3633448) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131500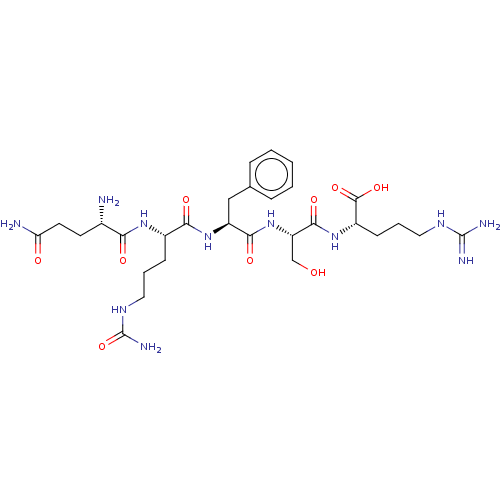 (CHEMBL3633451) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382927 (CHEMBL2029394) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human aminopeptidase N | ACS Med Chem Lett 3: 20-24 (2012) Article DOI: 10.1021/ml200182v BindingDB Entry DOI: 10.7270/Q2P84CZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382499 (CHEMBL2022225) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant neutral endopeptidase using Ala-AMC as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382499 (CHEMBL2022225) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant neprilysin using Abz-dR-G-L-EDDnp as substrate incubated for 10 mins prior to substrate addition measured for 40 mins... | ACS Med Chem Lett 3: 20-24 (2012) Article DOI: 10.1021/ml200182v BindingDB Entry DOI: 10.7270/Q2P84CZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382500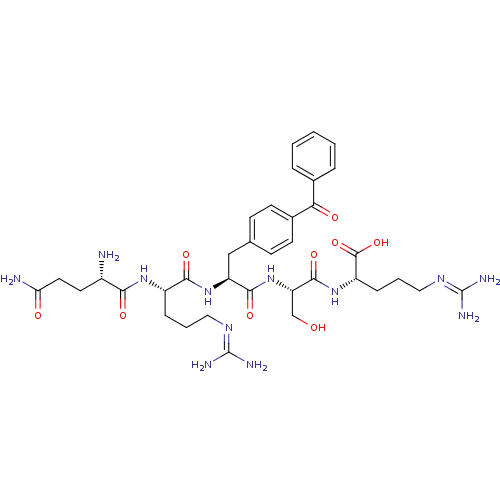 (CHEMBL2024270) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382505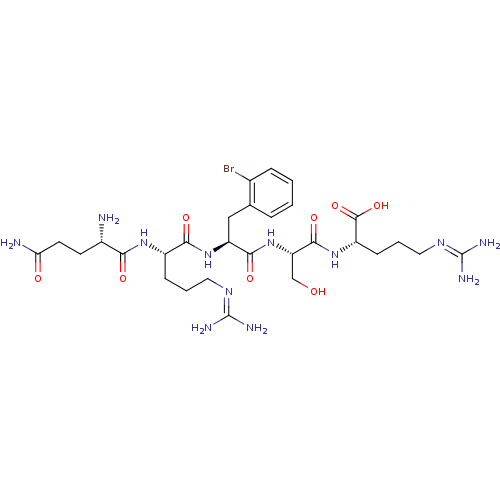 (CHEMBL2022232) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382510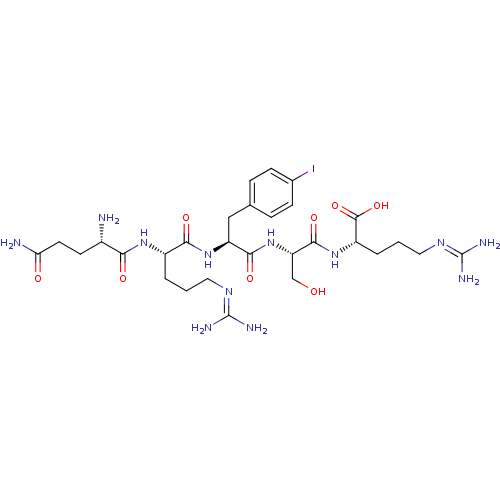 (CHEMBL2022236) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382499 (CHEMBL2022225) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131498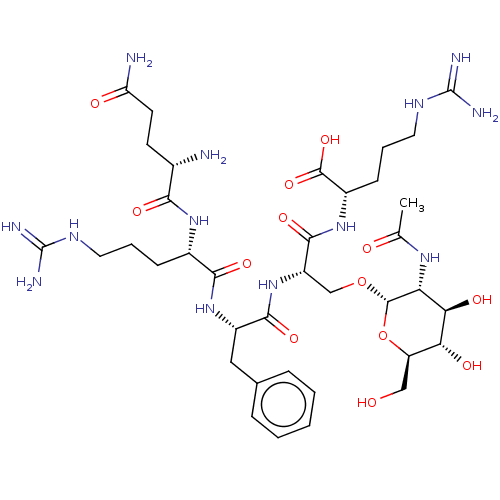 (CHEMBL3633453) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382503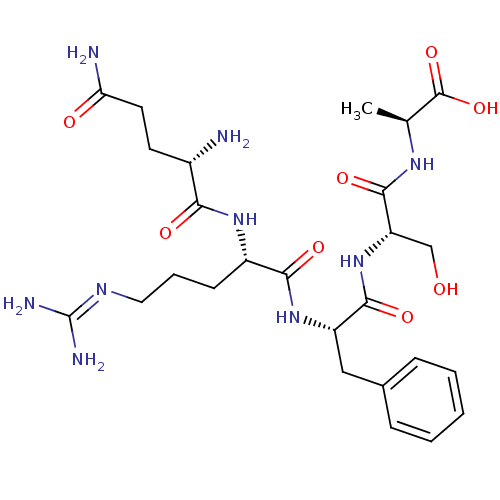 (CHEMBL2022230) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50131536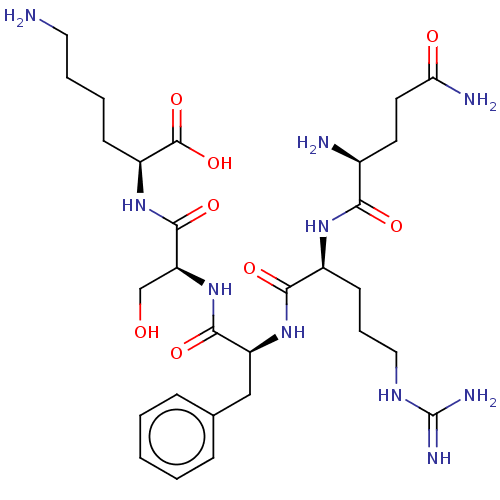 (CHEMBL3633450) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant neutral endopeptidase using Ala-AMC as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382519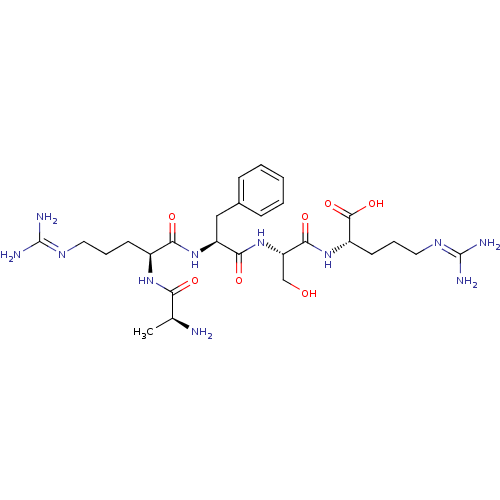 (CHEMBL2022226) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382502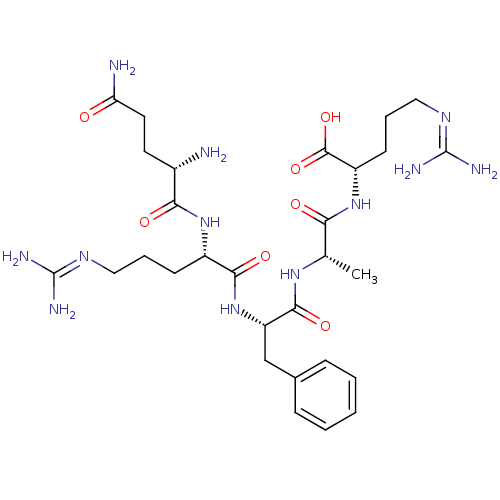 (CHEMBL2022229) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382504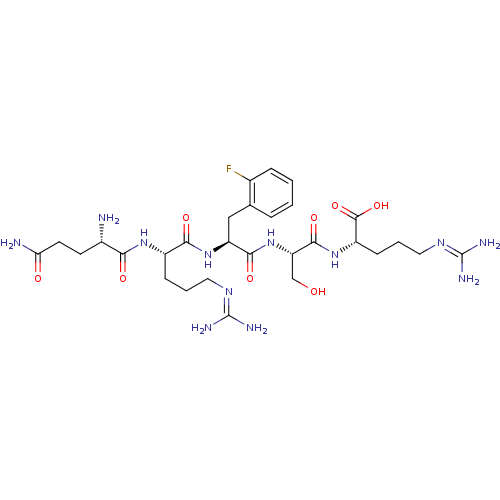 (CHEMBL2022231) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382517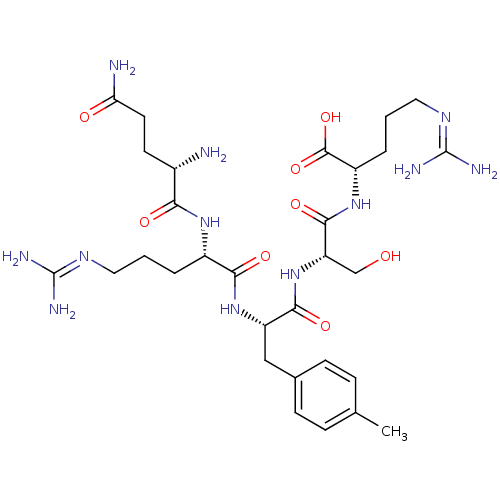 (CHEMBL2024269) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382502 (CHEMBL2022229) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382504 (CHEMBL2022231) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382509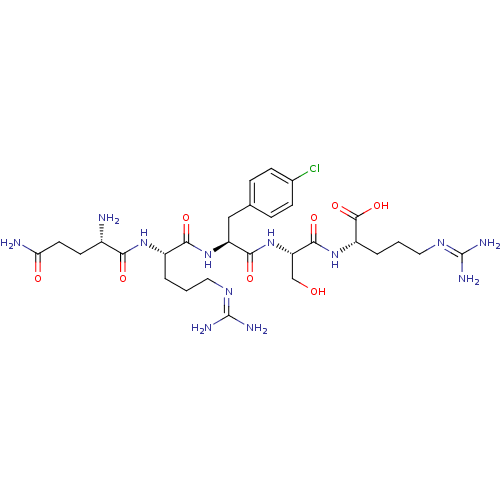 (CHEMBL2022235) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50131537 (CHEMBL3633449) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant neutral endopeptidase using Ala-AMC as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382925 (CHEMBL2029398) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant neprilysin using Abz-dR-G-L-EDDnp as substrate incubated for 10 mins prior to substrate addition measured for 40 mins... | ACS Med Chem Lett 3: 20-24 (2012) Article DOI: 10.1021/ml200182v BindingDB Entry DOI: 10.7270/Q2P84CZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131497 (CHEMBL3633454) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382514 (CHEMBL2024266) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131499 (CHEMBL3633452) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382516 (CHEMBL2024268) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382505 (CHEMBL2022232) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382520 (CHEMBL2022227) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382519 (CHEMBL2022226) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131537 (CHEMBL3633449) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382501 (CHEMBL2022228) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131536 (CHEMBL3633450) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382503 (CHEMBL2022230) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382509 (CHEMBL2022235) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382514 (CHEMBL2024266) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382513 (CHEMBL2022239) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant AP-N ectopeptidase activity using alanine-MCA as substrate preincubated for 10 mins with substrate by fluorimetric an... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50382516 (CHEMBL2024268) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant NEP ectopeptidase activity using Abz-dRGL-(EDDnp) as substrate preincubated for 10 mins with substrate by fluorimetri... | J Med Chem 55: 1181-8 (2012) Article DOI: 10.1021/jm2012112 BindingDB Entry DOI: 10.7270/Q2NC627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50382928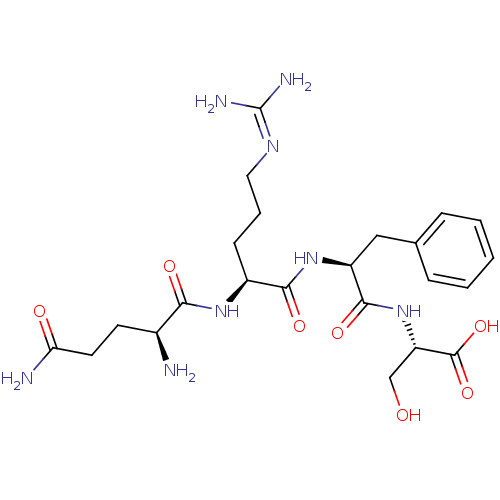 (CHEMBL2029395) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human aminopeptidase N | ACS Med Chem Lett 3: 20-24 (2012) Article DOI: 10.1021/ml200182v BindingDB Entry DOI: 10.7270/Q2P84CZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50131539 (CHEMBL3633447) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant aminopeptidase N using AbzdR-G-L-EDDnp as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50131500 (CHEMBL3633451) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Inhibition of human recombinant neutral endopeptidase using Ala-AMC as substrate after 20 to 40 mins by fluorometry | Bioorg Med Chem Lett 25: 5190-3 (2015) Article DOI: 10.1016/j.bmcl.2015.09.071 BindingDB Entry DOI: 10.7270/Q2FN182S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 82 total ) | Next | Last >> |