

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity to wild type human COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50491628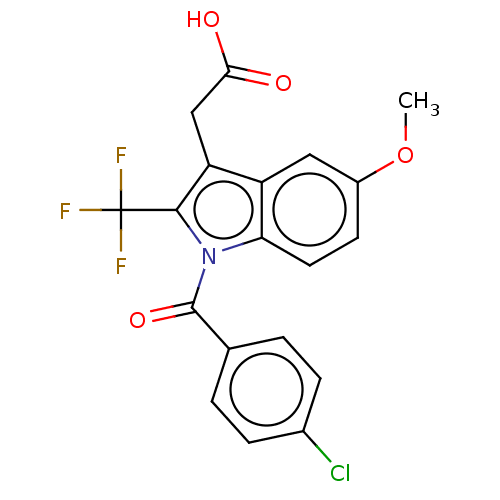 (CHEMBL2386352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Binding affinity to wild type human COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type ovine COX1 expressed in ram seminal vesicles using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC fol... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type mouse COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC followed by... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50491628 (CHEMBL2386352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of COX2 in human HNSCC1483 cells using [1-14C]-arachidonic acid as substrate assessed as inhibition of substrate oxygenation preincubated ... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type human COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC followed by... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50491628 (CHEMBL2386352) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type mouse COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC followed by... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50491628 (CHEMBL2386352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 388 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type human COX2 expressed in insect cells using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC followed by... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50491628 (CHEMBL2386352) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of wild type ovine COX1 expressed in ram seminal vesicles using [1-14C]-arachidonic acid as substrate incubated for 17 mins at 25 degC fol... | ACS Med Chem Lett 4: 486-490 (2013) Article DOI: 10.1021/ml400066a BindingDB Entry DOI: 10.7270/Q28D005G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50037874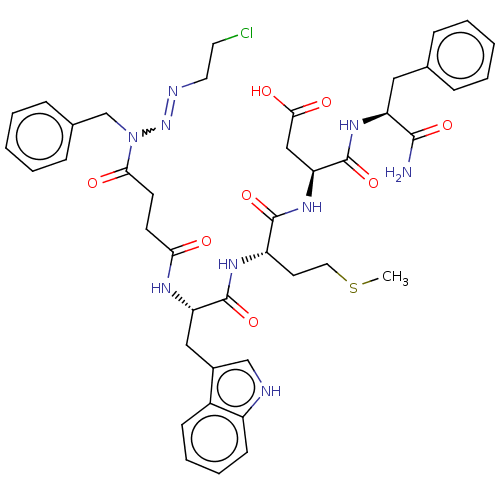 (CBS-5 | CHEMBL124944) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
NCI-Frederick Cancer Research and Development Center Curated by ChEMBL | Assay Description Tested for the 50% inhibition level against [125I]- gastrin binding to guinea pig gastric glands | J Med Chem 37: 3812-8 (1994) BindingDB Entry DOI: 10.7270/Q2TM7BRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50037873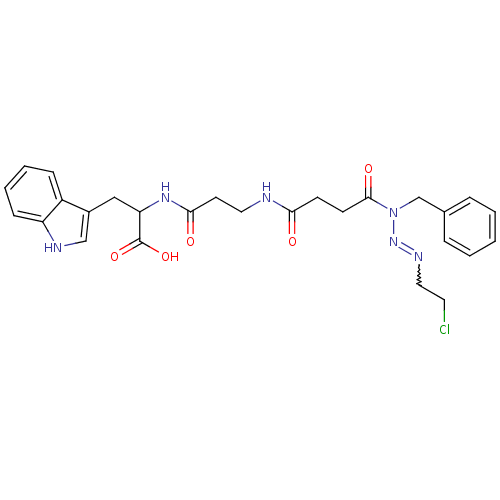 (CBS-2 | CHEMBL341130) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a |
NCI-Frederick Cancer Research and Development Center Curated by ChEMBL | Assay Description Tested for the 50% inhibition level against [125I]- gastrin binding in AR42J cells | J Med Chem 37: 3812-8 (1994) BindingDB Entry DOI: 10.7270/Q2TM7BRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50026690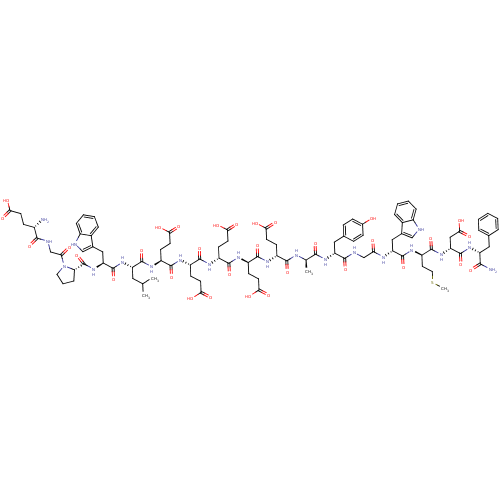 (2-[2-(2-{2-[2-{2-[2-(2-Amino-3-phenyl-propionylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Cancer Research and Development Center Curated by ChEMBL | Assay Description Tested in vitro for gastrin binding to gastrin receptors from guinea pig gastric glands | J Med Chem 37: 3812-8 (1994) BindingDB Entry DOI: 10.7270/Q2TM7BRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50026690 (2-[2-(2-{2-[2-{2-[2-(2-Amino-3-phenyl-propionylami...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick Cancer Research and Development Center Curated by ChEMBL | Assay Description Tested in vitro by the gastrin binding assay for the competitive binding with iodinated gastrin for the gastrin receptors in AR42J cells | J Med Chem 37: 3812-8 (1994) BindingDB Entry DOI: 10.7270/Q2TM7BRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50037875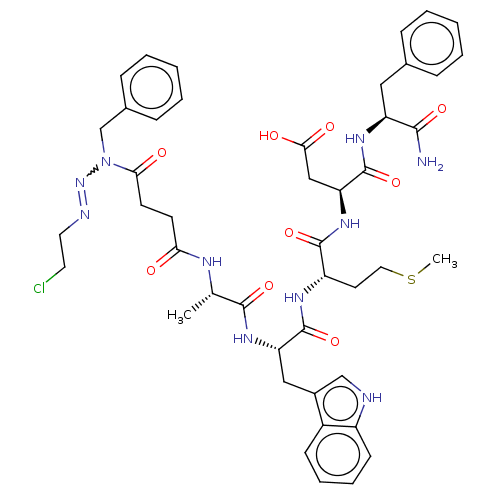 (CBS-4 | CHEMBL341330) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
NCI-Frederick Cancer Research and Development Center Curated by ChEMBL | Assay Description Tested for the 50% inhibition level against [125I]- gastrin binding to guinea pig gastric glands | J Med Chem 37: 3812-8 (1994) BindingDB Entry DOI: 10.7270/Q2TM7BRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50026690 (2-[2-(2-{2-[2-{2-[2-(2-Amino-3-phenyl-propionylami...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
NCI-Frederick Cancer Research and Development Center Curated by ChEMBL | Assay Description Tested for the 50% inhibition level against [125I]- gastrin binding in AR42J cells | J Med Chem 37: 3812-8 (1994) BindingDB Entry DOI: 10.7270/Q2TM7BRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||