 Found 194 hits with Last Name = 'shepherd' and Initial = 'c'
Found 194 hits with Last Name = 'shepherd' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50150074
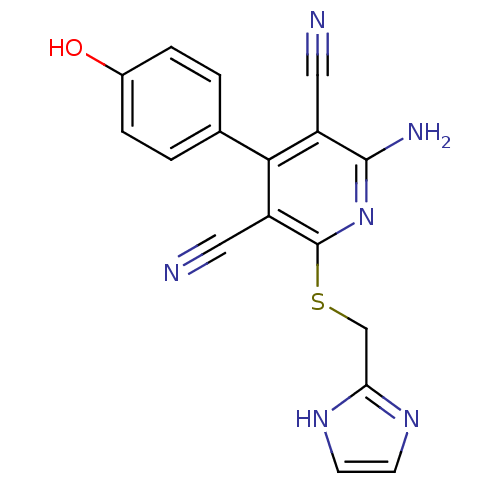
(2-Amino-4-(4-hydroxy-phenyl)-6-(1H-imidazol-2-ylme...)Show SMILES Nc1nc(SCc2ncc[nH]2)c(C#N)c(-c2ccc(O)cc2)c1C#N Show InChI InChI=1S/C17H12N6OS/c18-7-12-15(10-1-3-11(24)4-2-10)13(8-19)17(23-16(12)20)25-9-14-21-5-6-22-14/h1-6,24H,9H2,(H2,20,23)(H,21,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM82022
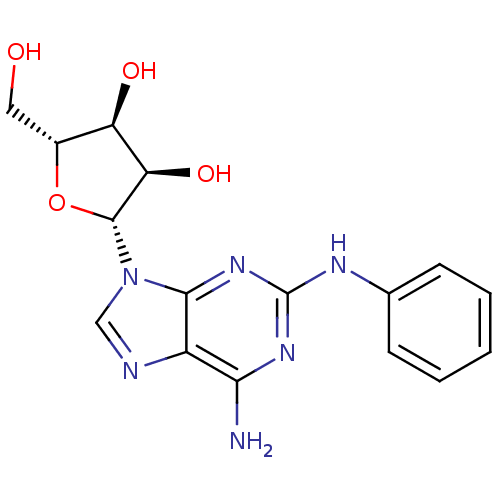
(2-(Phenylamino)ado (CV-1808) | 2-[6-Amino-2-(3-cyc...)Show SMILES Nc1nc(Nc2ccccc2)nc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C16H18N6O4/c17-13-10-14(21-16(20-13)19-8-4-2-1-3-5-8)22(7-18-10)15-12(25)11(24)9(6-23)26-15/h1-5,7,9,11-12,15,23-25H,6H2,(H3,17,19,20,21)/t9-,11-,12-,15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM14487

((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(18)6(17)4(1-16)19-10/h2-4,6-7,10,16-18H,1H2,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50157314
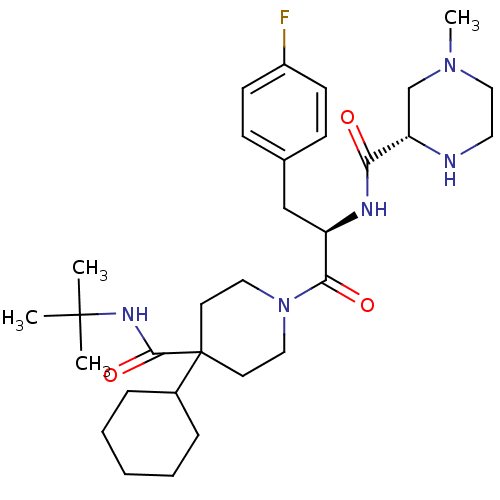
((S)-4-Methyl-piperazine-2-carboxylic acid [(R)-2-(...)Show SMILES CN1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50157314

((S)-4-Methyl-piperazine-2-carboxylic acid [(R)-2-(...)Show SMILES CN1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8392

(CHEMBL408564 | N-[5-bromo-6-(furan-2-yl)-1H-pyrazo...)Show InChI InChI=1S/C14H11BrN4O2/c15-9-6-8-12(16-11(9)10-2-1-5-21-10)18-19-13(8)17-14(20)7-3-4-7/h1-2,5-7H,3-4H2,(H2,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027107

(CHEMBL2145455)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(C)CN1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C34H54FN5O3/c1-24(2)22-39-20-17-36-29(23-39)30(41)37-28(21-25-11-13-27(35)14-12-25)31(42)40-18-15-34(16-19-40,26-9-7-6-8-10-26)32(43)38-33(3,4)5/h11-14,24,26,28-29,36H,6-10,15-23H2,1-5H3,(H,37,41)(H,38,43)/t28-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027131

(CHEMBL2145454)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(C)(C)NC(=O)C1(CCN(CC1)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@@H]1CN(CC(F)F)CCN1)C1CCCCC1 Show InChI InChI=1S/C32H48F3N5O3/c1-31(2,3)38-30(43)32(23-7-5-4-6-8-23)13-16-40(17-14-32)29(42)25(19-22-9-11-24(33)12-10-22)37-28(41)26-20-39(18-15-36-26)21-27(34)35/h9-12,23,25-27,36H,4-8,13-21H2,1-3H3,(H,37,41)(H,38,43)/t25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50157320

((S)-1-Methyl-piperazine-2-carboxylic acid [(R)-2-(...)Show SMILES CC(C)(C)NC(=O)C1(CCN(CC1)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@@H]1CNCCN1C(=O)OC(C)(C)C)C1CCCCC1 Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-33-16-19-36(26)4/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50029747
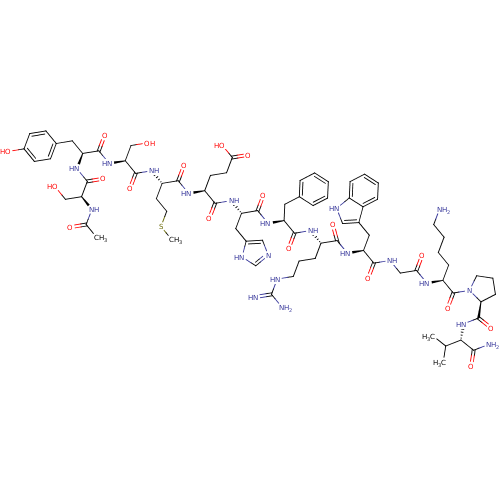
((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50157321

((R)-4-Methyl-piperazine-2-carboxylic acid [(R)-2-(...)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CCN1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027156

(CHEMBL2145456)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.C[C@H](N)C(=O)N1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C33H51FN6O4/c1-22(35)29(42)40-19-16-36-27(21-40)28(41)37-26(20-23-10-12-25(34)13-11-23)30(43)39-17-14-33(15-18-39,24-8-6-5-7-9-24)31(44)38-32(2,3)4/h10-13,22,24,26-27,36H,5-9,14-21,35H2,1-4H3,(H,37,41)(H,38,44)/t22-,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027108
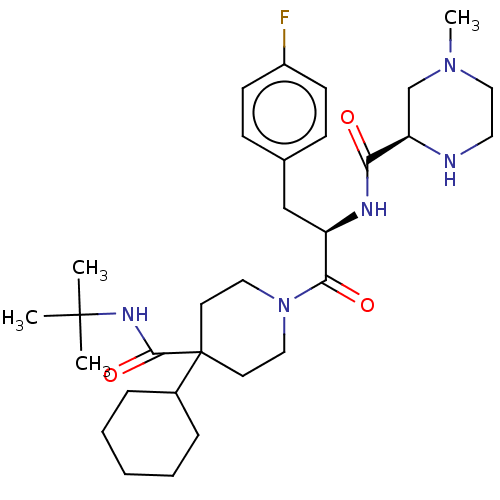
(CHEMBL3216179)Show SMILES Cl.Cl.CN1CCN[C@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50157315
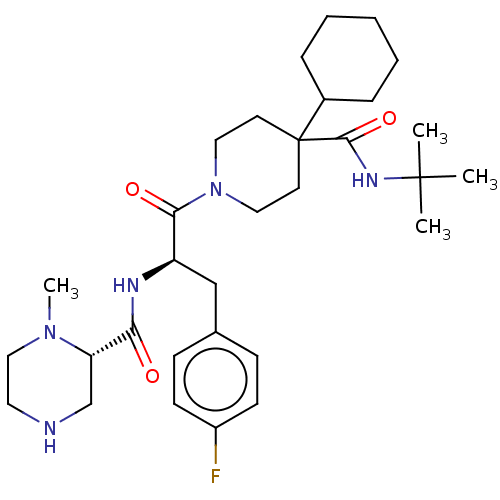
((S)-4-Methyl-piperazine-2-carboxylic acid [(S)-2-(...)Show SMILES Cl.Cl.CN1CCNC[C@H]1C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8246
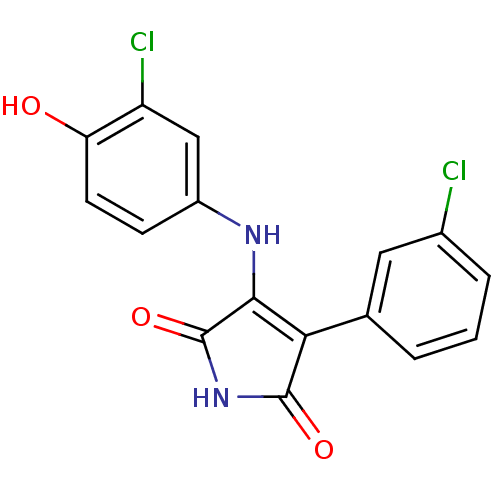
(3-[(3-chloro-4-hydroxyphenyl)amino]-4-(3-chlorophe...)Show SMILES Oc1ccc(NC2=C(C(=O)NC2=O)c2cccc(Cl)c2)cc1Cl |t:6| Show InChI InChI=1S/C16H10Cl2N2O3/c17-9-3-1-2-8(6-9)13-14(16(23)20-15(13)22)19-10-4-5-12(21)11(18)7-10/h1-7,21H,(H2,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50595384
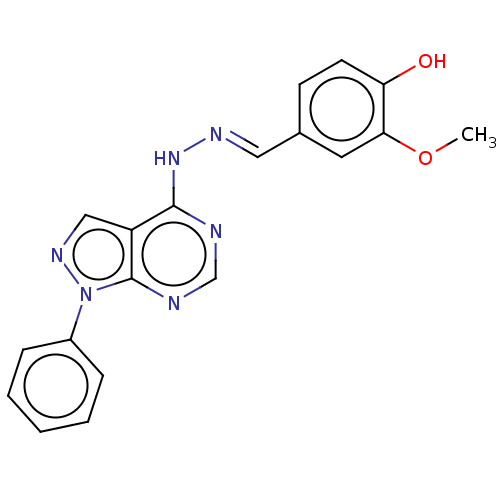
(CHEMBL63803)Show SMILES COc1cc(\C=N\Nc2ncnc3n(ncc23)-c2ccccc2)ccc1O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50520329
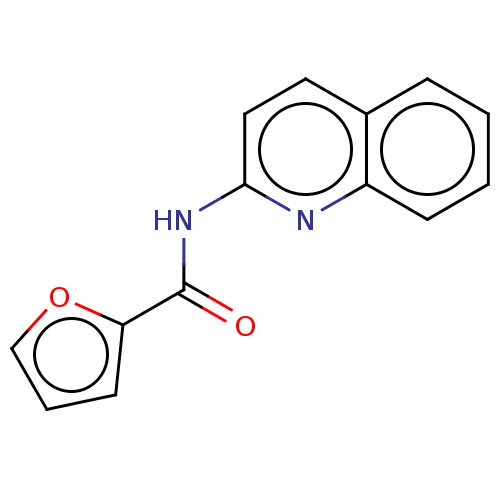
(CHEMBL4550896)Show InChI InChI=1S/C14H10N2O2/c17-14(12-6-3-9-18-12)16-13-8-7-10-4-1-2-5-11(10)15-13/h1-9H,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00099
BindingDB Entry DOI: 10.7270/Q23200WW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547407
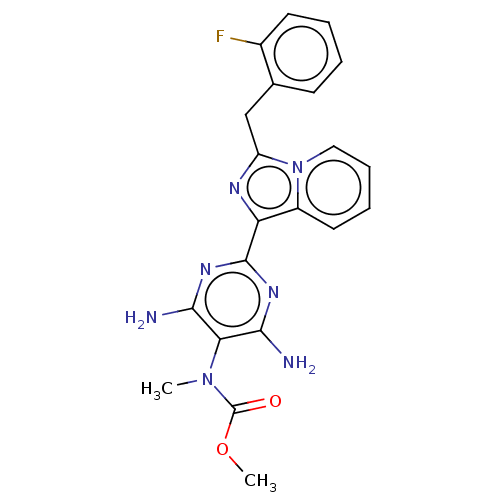
(CHEMBL4751262)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-c1nc(Cc2ccccc2F)n2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547430

(CHEMBL4753045)Show SMILES CC1(C)C(=O)Nc2nc(nc(N)c12)-n1nc(Cc2c(F)ccc(F)c2F)c2cc(Cl)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547428

(CHEMBL4761962)Show SMILES CC1(C)C(=O)Nc2nc(nc(N)c12)-n1nc(Cc2ccccc2F)c2cc(Cl)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50157314

((S)-4-Methyl-piperazine-2-carboxylic acid [(R)-2-(...)Show SMILES CN1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-1 receptor expressed in CHO cells using [125I]-NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547429

(CHEMBL4755974)Show SMILES CC1(C)C(=O)Nc2nc(nc(N)c12)-n1nc(Cc2c(F)ccc(F)c2F)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50157314

((S)-4-Methyl-piperazine-2-carboxylic acid [(R)-2-(...)Show SMILES CN1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-3 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50157314

((S)-4-Methyl-piperazine-2-carboxylic acid [(R)-2-(...)Show SMILES CN1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-5 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50157314

((S)-4-Methyl-piperazine-2-carboxylic acid [(R)-2-(...)Show SMILES CN1CCN[C@@H](C1)C(=O)N[C@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-5 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547417

(CHEMBL4793885)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2cc(F)ccc2F)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547420

(CHEMBL4781791)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2c(F)ccc(F)c2F)c2cc(Cl)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547436

(CHEMBL4788234)Show SMILES CC1(C)C(=O)Nc2nc(nc(N)c12)-n1nc(CCCC(F)(F)F)c2cc(Cl)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547427

(CHEMBL4789308)Show SMILES CC1(C)C(=O)Nc2nc(nc(N)c12)-n1nc(Cc2ccccc2F)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547412
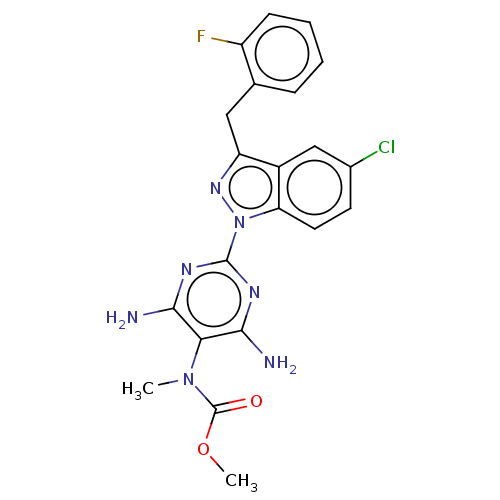
(CHEMBL4751235)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2ccccc2F)c2cc(Cl)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50027155
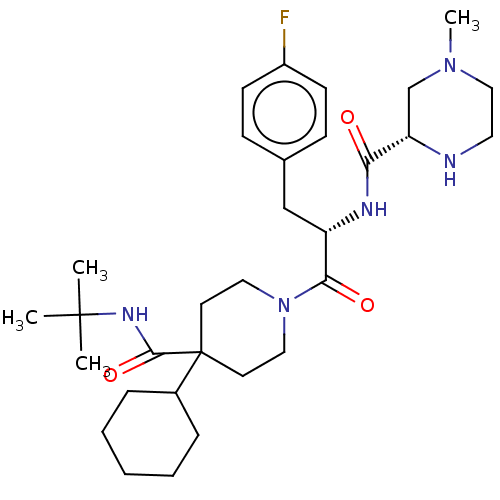
(CHEMBL3215542)Show SMILES Cl.Cl.CN1CCN[C@@H](C1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N1CCC(CC1)(C1CCCCC1)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C31H48FN5O3/c1-30(2,3)35-29(40)31(23-8-6-5-7-9-23)14-17-37(18-15-31)28(39)25(20-22-10-12-24(32)13-11-22)34-27(38)26-21-36(4)19-16-33-26/h10-13,23,25-26,33H,5-9,14-21H2,1-4H3,(H,34,38)(H,35,40)/t25-,26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Concentration for 50% inhibition of human melanocortin-4 receptor expressed in CHO cells using [125I]NDP-alpha-MSH as radioligand |
Bioorg Med Chem Lett 15: 171-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.020
BindingDB Entry DOI: 10.7270/Q2G44R2V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50547435

(CHEMBL4758823)Show SMILES CC1(C)C(=O)Nc2nc(nc(N)c12)-n1nc(CCC(F)(F)F)c2cc(Cl)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547416

(CHEMBL4759918)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2cccc(F)c2F)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547414
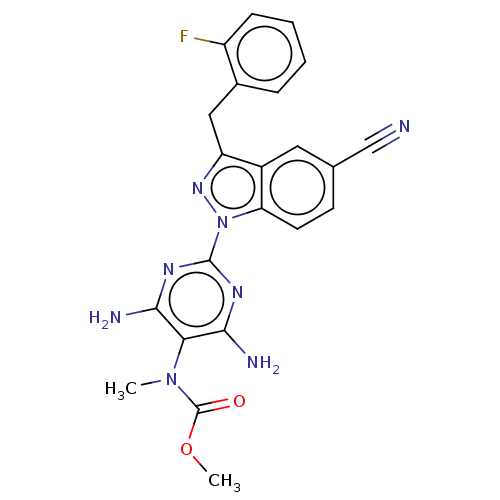
(CHEMBL4751496)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2ccccc2F)c2cc(ccc12)C#N | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547433
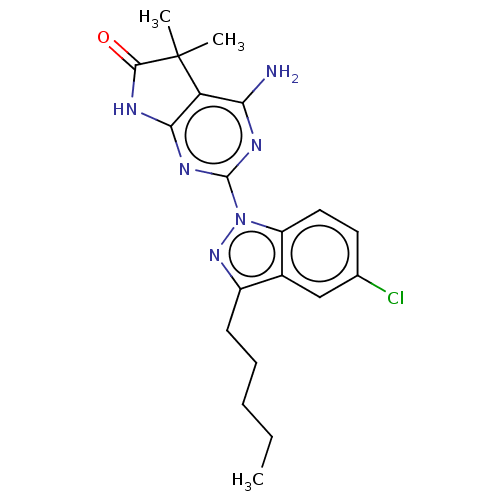
(CHEMBL4740488)Show SMILES CCCCCc1nn(-c2nc3NC(=O)C(C)(C)c3c(N)n2)c2ccc(Cl)cc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547431
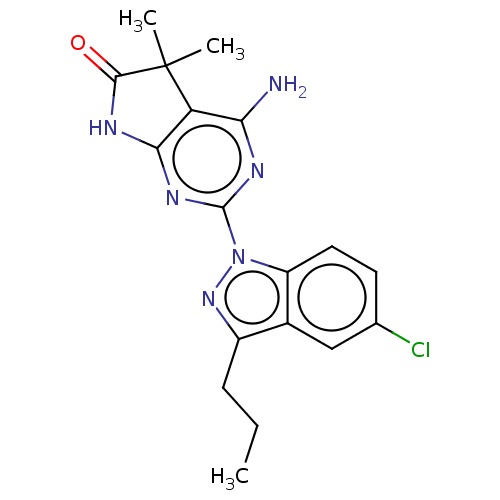
(CHEMBL4778917)Show SMILES CCCc1nn(-c2nc3NC(=O)C(C)(C)c3c(N)n2)c2ccc(Cl)cc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547415
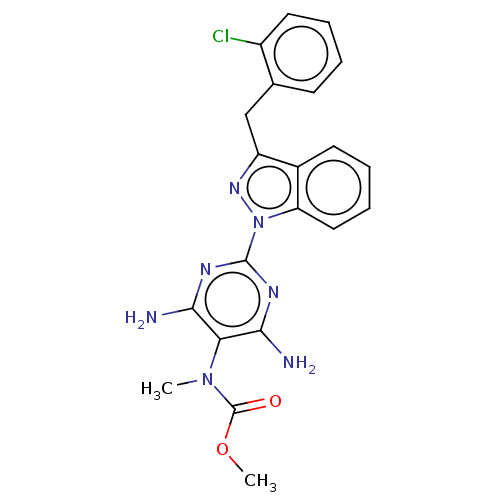
(CHEMBL4779699)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2ccccc2Cl)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547432
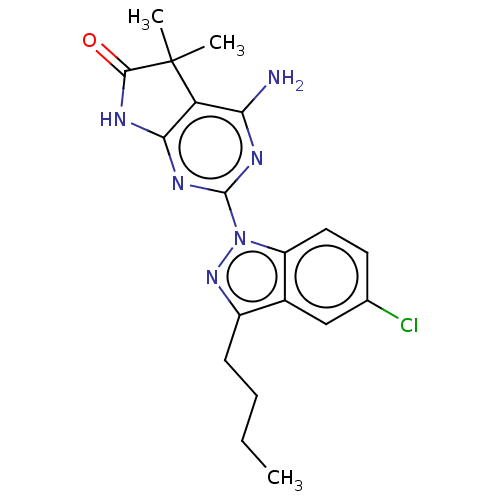
(CHEMBL4752891)Show SMILES CCCCc1nn(-c2nc3NC(=O)C(C)(C)c3c(N)n2)c2ccc(Cl)cc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547422
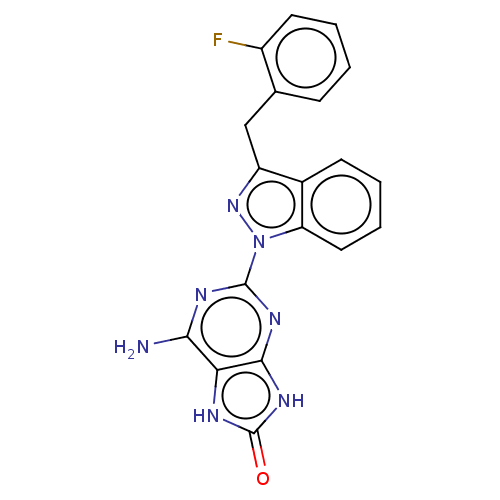
(CHEMBL4762232)Show SMILES Nc1nc(nc2[nH]c(=O)[nH]c12)-n1nc(Cc2ccccc2F)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547413

(CHEMBL4787520)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2ccccc2F)c2cc(F)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547408

(CHEMBL4763259)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-c1nc(Cc2ccccc2F)n2ncccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547418

(CHEMBL4740085)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2cc(F)cc(F)c2F)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547411

(CHEMBL4793528)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2ccccc2F)c2cc(OC)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547409

(CHEMBL4752570)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2ccccc2F)c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50547410

(CHEMBL4785740)Show SMILES COC(=O)N(C)c1c(N)nc(nc1N)-n1nc(Cc2ccccc2F)c2cc(C)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50547435

(CHEMBL4758823)Show SMILES CC1(C)C(=O)Nc2nc(nc(N)c12)-n1nc(CCC(F)(F)F)c2cc(Cl)ccc12 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50547435

(CHEMBL4758823)Show SMILES CC1(C)C(=O)Nc2nc(nc(N)c12)-n1nc(CCC(F)(F)F)c2cc(Cl)ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127574
BindingDB Entry DOI: 10.7270/Q2XG9VRF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data