

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50249180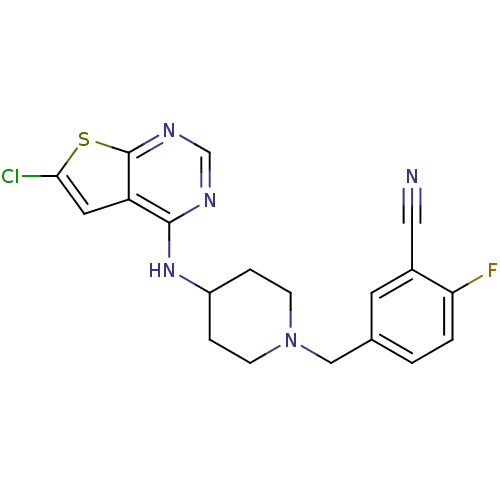 (5-((4-(6-chlorothieno[2,3-d]pyrimidin-4-ylamino)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Inhibition of 5-HT2B receptor (unknown origin) | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50510877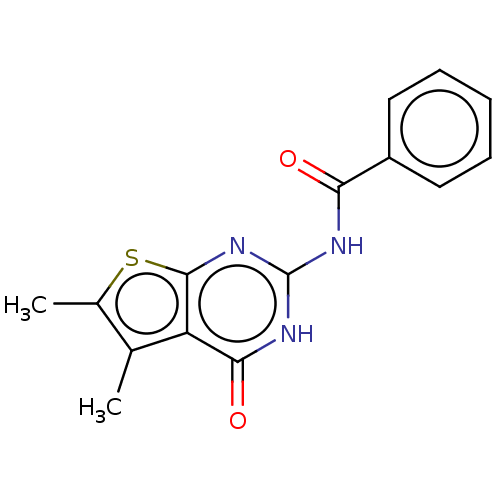 (CHEMBL4530036) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-HEMADO from human A3 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50510879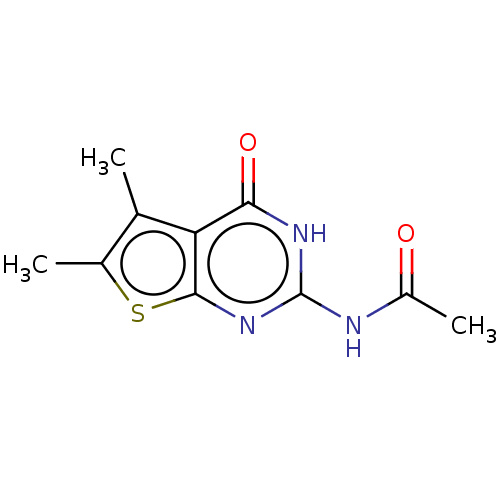 (CHEMBL4548341) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 524 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-HEMADO from human A3 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50510877 (CHEMBL4530036) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human A2A receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50510875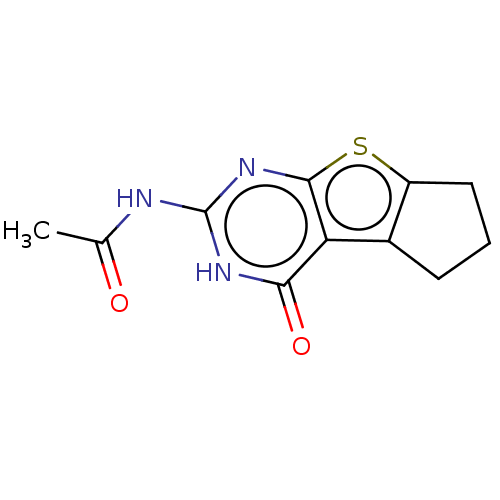 (CHEMBL4447927) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-HEMADO from human A3 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50510878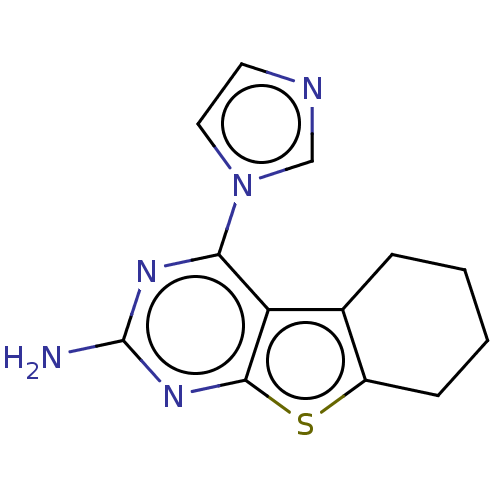 (CHEMBL4552737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human A2A receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50510876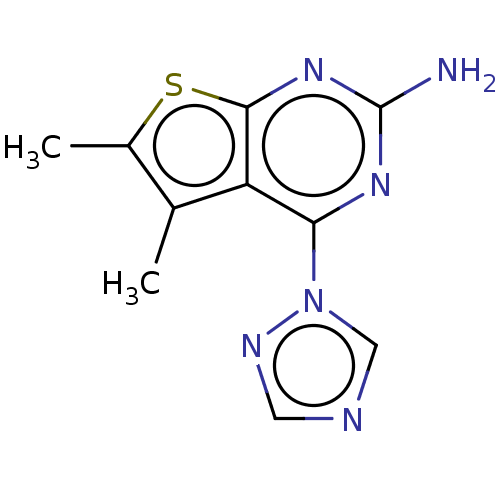 (CHEMBL4555340) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human A2A receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50510878 (CHEMBL4552737) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA binding from human A1 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50510878 (CHEMBL4552737) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-HEMADO from human A3 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50510875 (CHEMBL4447927) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human A2A receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50510877 (CHEMBL4530036) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA binding from human A1 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50510876 (CHEMBL4555340) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-HEMADO from human A3 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50510879 (CHEMBL4548341) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA binding from human A1 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50510879 (CHEMBL4548341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-NECA from human A2A receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50510876 (CHEMBL4555340) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA binding from human A1 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50510875 (CHEMBL4447927) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Displacement of [3H]-CCPA binding from human A1 receptor expressed in CHO cells by radioligand competitive binding assay | Bioorg Med Chem 27: 1159-1194 (2019) Article DOI: 10.1016/j.bmc.2019.02.044 BindingDB Entry DOI: 10.7270/Q28055X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554464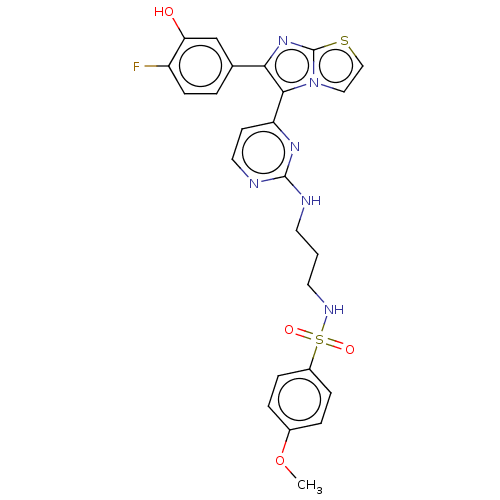 (US11332479, Compound 50III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585092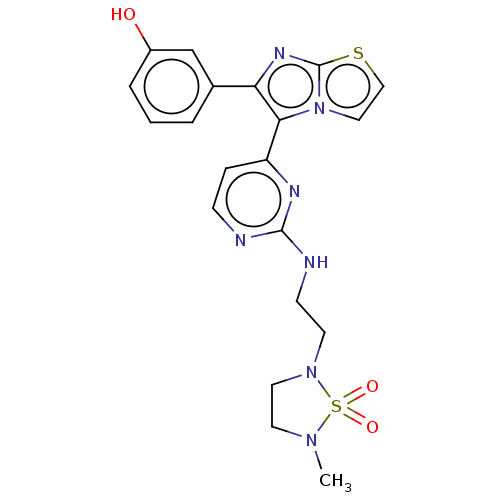 (CHEMBL5087577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase ROS (Homo sapiens (Human)) | BDBM50059889 ((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyung Hee University Curated by ChEMBL | Assay Description Inhibition of ROS1 by HotSpot assay relative to control | Bioorg Med Chem Lett 19: 4720-3 (2009) Article DOI: 10.1016/j.bmcl.2009.06.066 BindingDB Entry DOI: 10.7270/Q2Z89CG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554470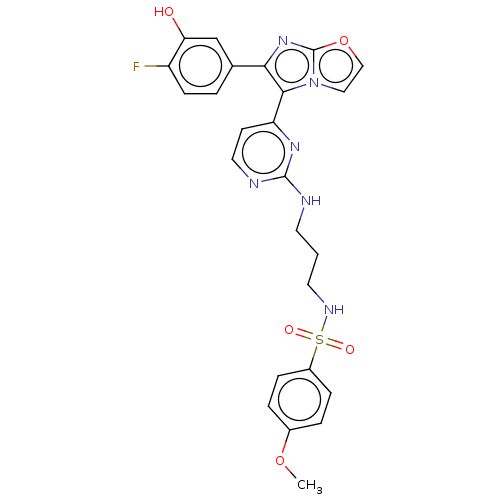 (US11332479, Compound 72IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554469 (US11332479, Compound 70IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554462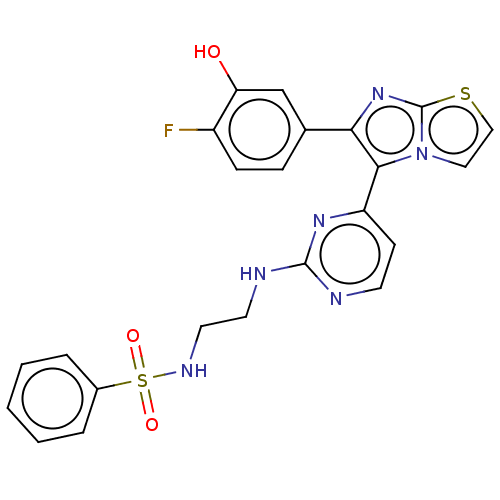 (US11332479, Compound 46III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554471 (US11332479, Compound 73IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554463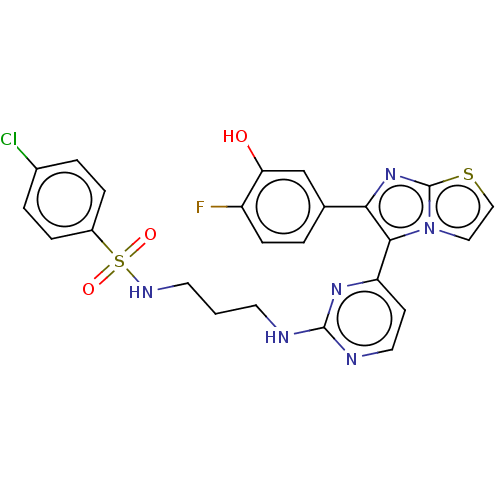 (US11332479, Compound 48III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM221688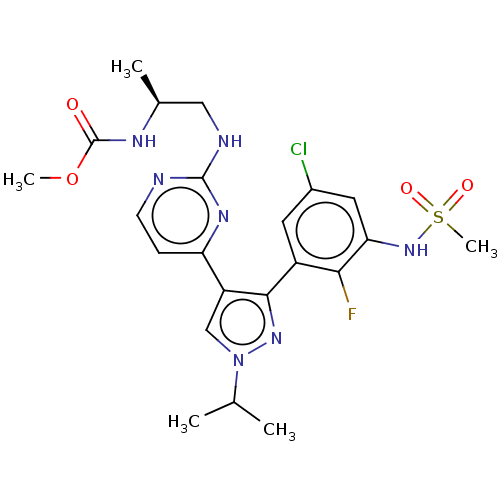 (US10568884, Cpd 9 | US9314464, 9 | US9593100, Comp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science & Technology (UST) Curated by ChEMBL | Assay Description Inhibition of BRAF V600E mutant (unknown origin) | Eur J Med Chem 158: 144-166 (2018) Article DOI: 10.1016/j.ejmech.2018.09.005 BindingDB Entry DOI: 10.7270/Q208682V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554460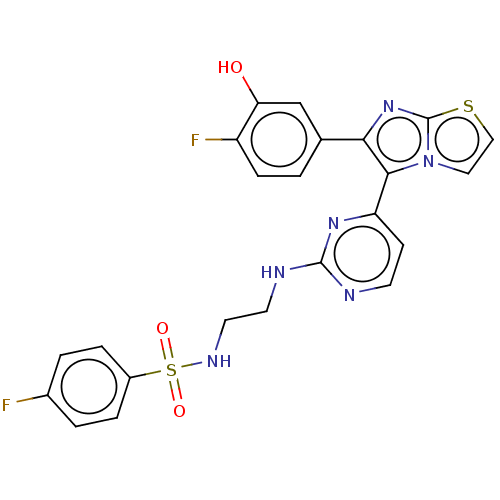 (US11332479, Compound 42III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554467 (US11332479, Compound 64IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554468 (US11332479, Compound 65IV) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50457446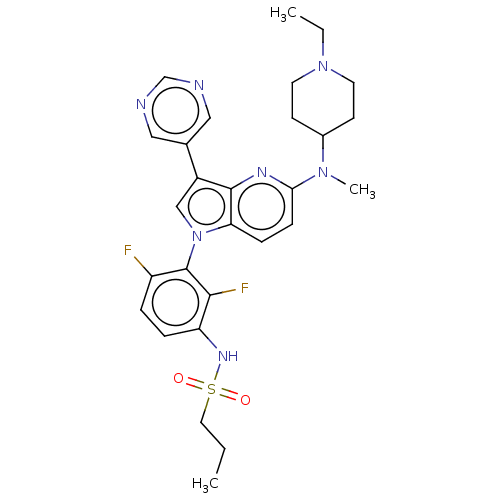 (CHEMBL4212692) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Centre (NRC ID: 60014618) Curated by ChEMBL | Assay Description Inhibition of BRAF (unknown origin) | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115493 BindingDB Entry DOI: 10.7270/Q2VX0M3G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506637 (CHEMBL4476226) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506637 (CHEMBL4476226) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of human AXL using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assay | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50428286 (DABRAFENIB | GSK2118436A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF V600E mutant (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127478 BindingDB Entry DOI: 10.7270/Q2862M5M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50428286 (DABRAFENIB | GSK2118436A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF V600E mutant (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113277 BindingDB Entry DOI: 10.7270/Q2VX0M8Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [696-1022,L858R] (Homo sapiens (Human)) | BDBM435718 (US10570155, Compound 2II | US11332479, Compound 2I...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50506648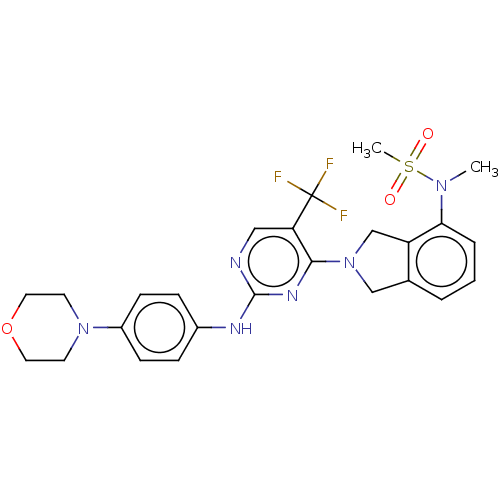 (CHEMBL4445940) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ... | Bioorg Med Chem Lett 28: 3761-3765 (2018) Article DOI: 10.1016/j.bmcl.2018.10.013 BindingDB Entry DOI: 10.7270/Q23T9MHD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554457 (US11332479, Compound 31II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435722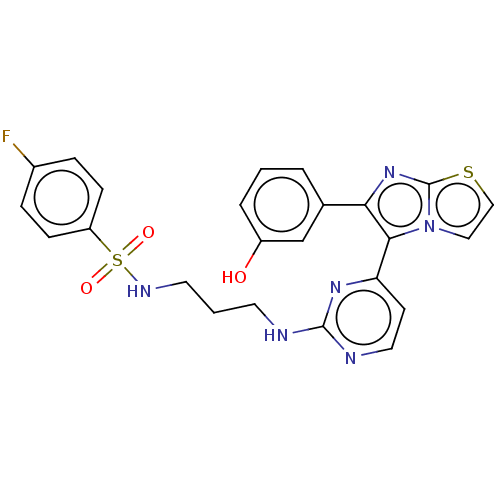 (US10570155, Compound 31III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50585081 (CHEMBL5086749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM435721 (US10570155, Compound 25III | US11332479, Compound ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY US Patent | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | US Patent US10570155 (2020) BindingDB Entry DOI: 10.7270/Q2N300B1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf [V600E] (Homo sapiens (Human)) | BDBM554465 (US11332479, Compound 52III) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (... | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ93MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAF proto-oncogene serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM50585092 (CHEMBL5087577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human wild type CRAF using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation count... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00230 BindingDB Entry DOI: 10.7270/Q2RV0SMN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM50460035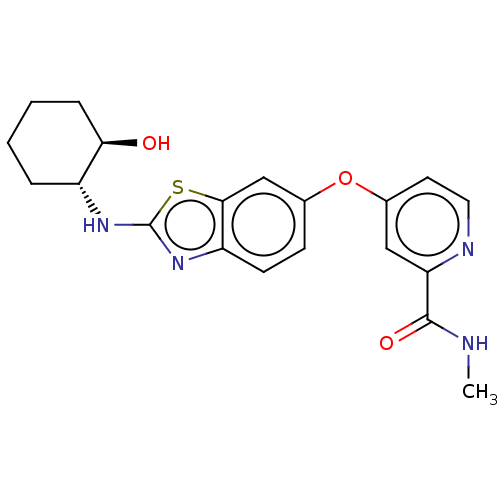 (CHEMBL4227505) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah Curated by ChEMBL | Assay Description Inhibition of CSF1R (unknown origin) | J Med Chem 61: 5450-5466 (2018) Article DOI: 10.1021/acs.jmedchem.7b00873 BindingDB Entry DOI: 10.7270/Q2P55R5S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50560548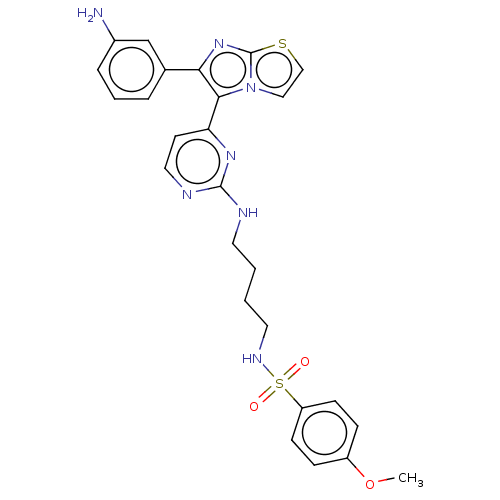 (CHEMBL4795335) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRAF V600E mutant (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127478 BindingDB Entry DOI: 10.7270/Q2862M5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 629 total ) | Next | Last >> |