

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50464147 (CHEMBL256907) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]MIP-1alpha from CCR5 receptor in rhesus monkey membrane incubated for 120 mins by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50334986 (4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1alpha from CCR5 in rhesus monkey Chem-1 cell membranes after 120 mins by liquid scintillation counting analysis | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from human mu opioid receptor expressed in CHO cells after 1 hr | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50392801 (CHEMBL2151247) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292920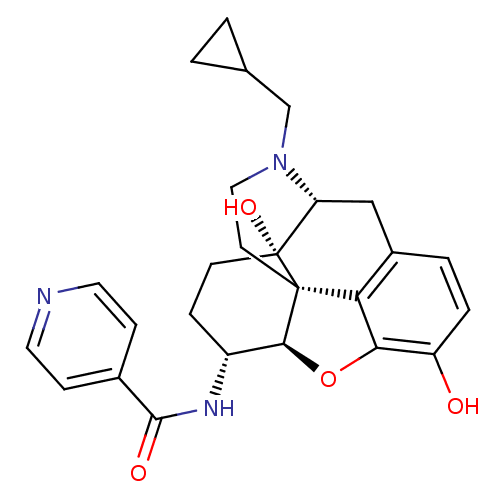 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity to wild type MOR (unknown origin) expressed in CHO cells after 15 mins by Ca2+ mobilization assay | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50558602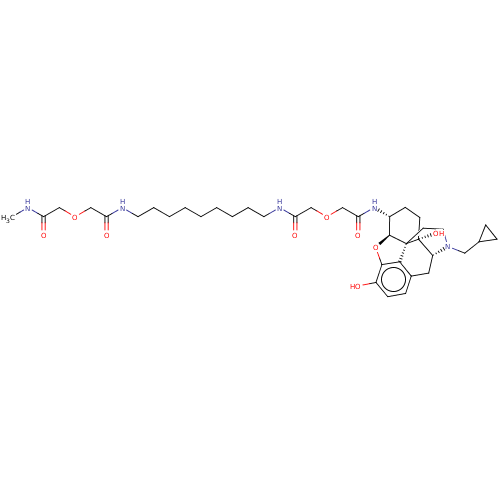 (CHEMBL4741368) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity to wild type MOR (unknown origin) expressed in CHO cells after 15 mins by Ca2+ mobilization assay | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292919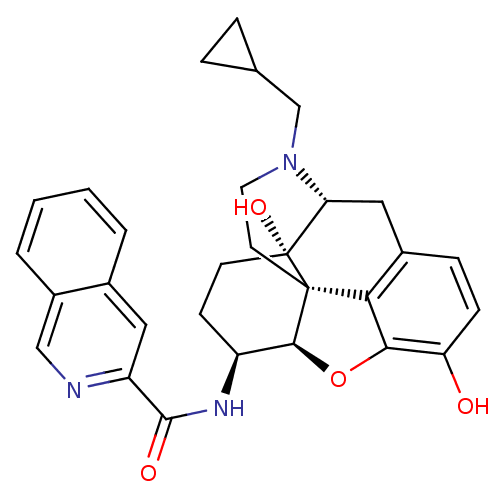 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity to wild type MOR (unknown origin) expressed in CHO cells after 15 mins by Ca2+ mobilization assay | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50558604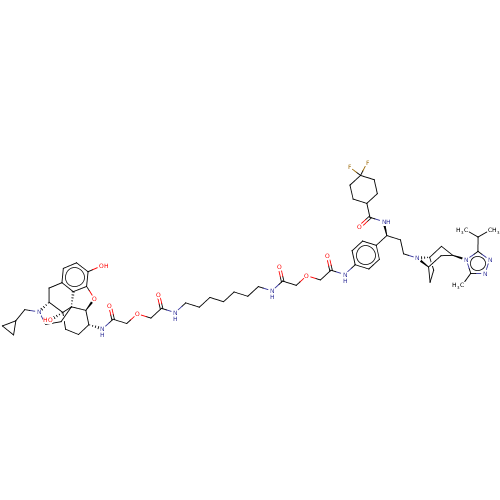 (CHEMBL4748891) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50558605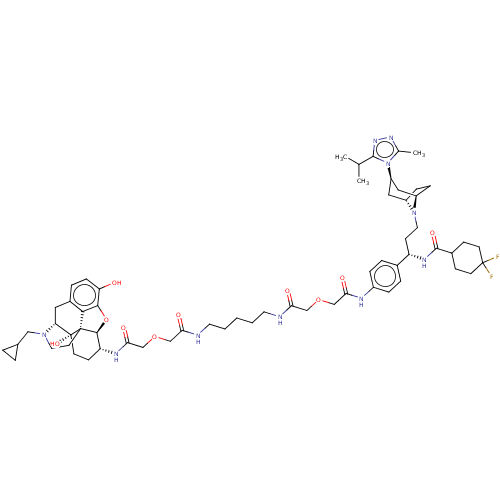 (CHEMBL4797451) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50558603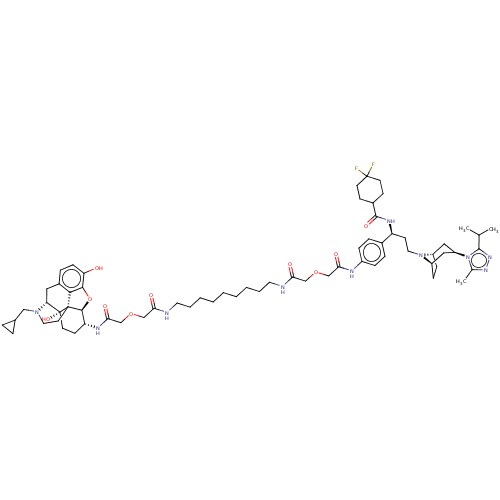 (CHEMBL4761986) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50496848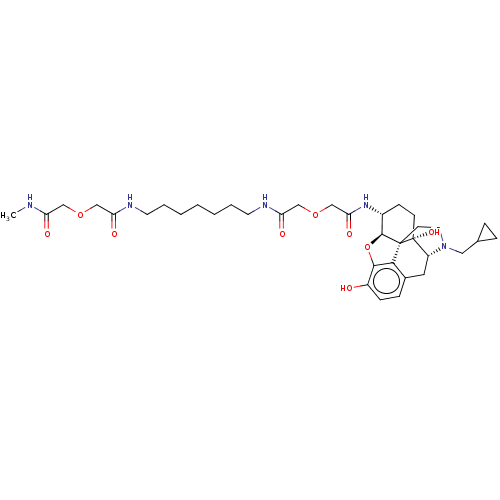 (CHEMBL3219778) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from human mu opioid receptor expressed in CHO cells after 1 hr | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50496848 (CHEMBL3219778) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (MOUSE) | BDBM50558601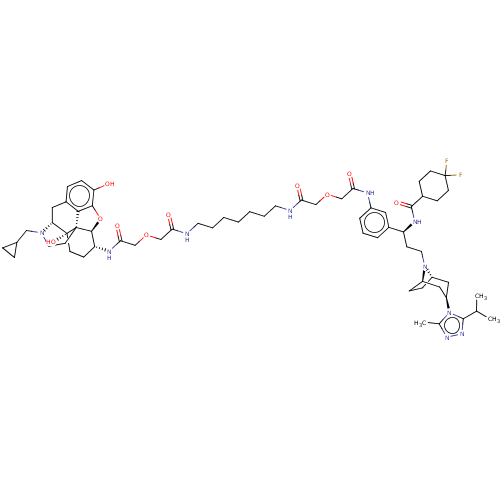 (CHEMBL4761273) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]NLX from mouse MOR expressed in CHO cell membrane incubated for 90 mins by liquid scintillation spectrophotometry | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50496850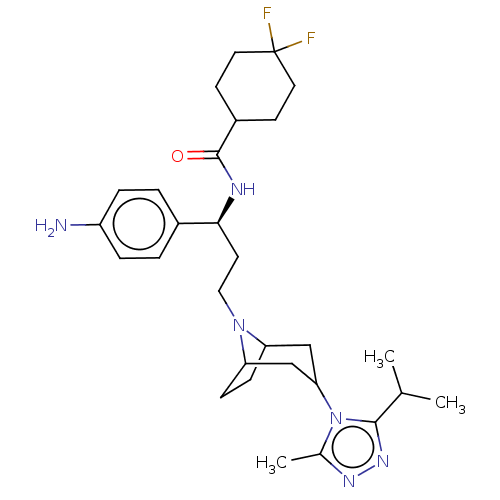 (CHEMBL3219780) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1alpha from CCR5 in rhesus monkey Chem-1 cell membranes after 120 mins by liquid scintillation counting analysis | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM160931 (US9107954, 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [3H]-naloxone from human mu opioid receptor expressed in CHO cells after 1 hr | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50496849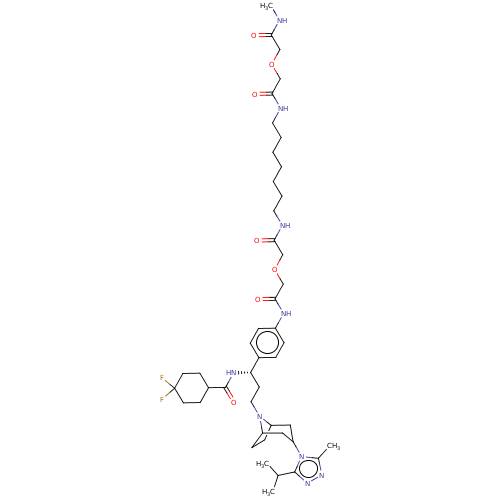 (CHEMBL3219779) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1alpha from CCR5 in rhesus monkey Chem-1 cell membranes after 120 mins by liquid scintillation counting analysis | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50558608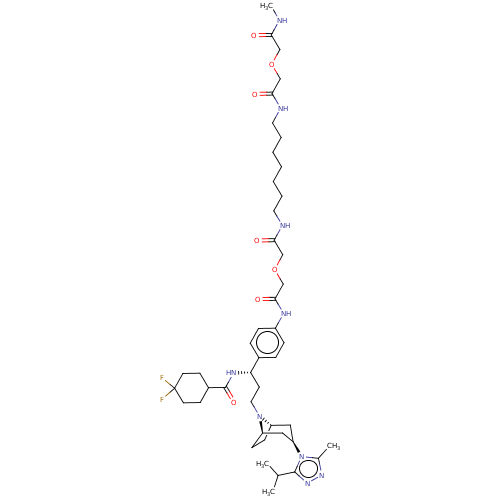 (CHEMBL4795179) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]MIP-1alpha from CCR5 receptor in rhesus monkey membrane incubated for 120 mins by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM50558604 (CHEMBL4748891) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]MIP-1alpha from CCR5 receptor in rhesus monkey membrane incubated for 120 mins by liquid scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Macaca fascicularis) | BDBM160931 (US9107954, 1) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Displacement of [125I]-MIP-1alpha from CCR5 in rhesus monkey Chem-1 cell membranes after 120 mins by liquid scintillation counting analysis | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50351152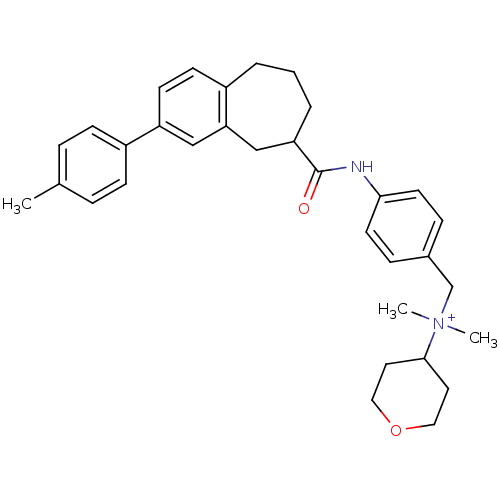 (CHEMBL1817917) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of CCL5 binding to CCR5 expressed in CHO cells | Bioorg Med Chem Lett 21: 5159-63 (2011) Article DOI: 10.1016/j.bmcl.2011.07.058 BindingDB Entry DOI: 10.7270/Q2MP53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50464147 (CHEMBL256907) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 receptor in human MOLT4 cells transfected with Gqi5 assessed as inhibition of RANTES-induced calcium mobilization incubat... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50334986 (4,4-Difluoro-cyclohexanecarboxylic acid {(S)-3-[(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in Gqi5 transfected human MOLT4 cells assessed as inhibition of RANTES-stimulated Ca2+ influx preincubated for 15 mins fo... | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292920 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity to wild type MOR (unknown origin) expressed in CHO cells after 15 mins by Ca2+ mobilization assay | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity to wild type MOR (unknown origin) expressed in CHO cells after 15 mins by Ca2+ mobilization assay | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292919 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Binding affinity to wild type MOR (unknown origin) expressed in CHO cells after 15 mins by Ca2+ mobilization assay | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50351152 (CHEMBL1817917) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at human CCR5 receptor expressed in MOLT4/CCR5 cells assessed as inhibition of CCL5-induced intracellular calcium mobilization by... | Bioorg Med Chem Lett 21: 5159-63 (2011) Article DOI: 10.1016/j.bmcl.2011.07.058 BindingDB Entry DOI: 10.7270/Q2MP53NR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in Gqi5 transfected CHO cells assessed as inhibition of DAMGO-stimulated Ca2+ influx preinc... | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50496850 (CHEMBL3219780) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in Gqi5 transfected human MOLT4 cells assessed as inhibition of RANTES-stimulated Ca2+ influx preincubated for 15 mins fo... | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50558601 (CHEMBL4761273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292920 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at MOR (unknown origin) assessed as inhibition of DAMGO-induced agonism | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50558605 (CHEMBL4797451) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50558603 (CHEMBL4761986) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50496848 (CHEMBL3219778) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in Gqi5 transfected CHO cells assessed as inhibition of DAMGO-stimulated Ca2+ influx preinc... | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50496848 (CHEMBL3219778) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM160931 (US9107954, 1) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in Gqi5 transfected CHO cells assessed as inhibition of DAMGO-stimulated Ca2+ influx preinc... | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50558604 (CHEMBL4748891) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50558602 (CHEMBL4741368) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50392801 (CHEMBL2151247) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human MOR transfected in CHO cells co-transfected with Gqi5 assessed as inhibition of DAMGO-induced calcium mobilization incub... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM160931 (US9107954, 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in Gqi5 transfected human MOLT4 cells assessed as inhibition of RANTES-stimulated Ca2+ influx preincubated for 15 mins fo... | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50558604 (CHEMBL4748891) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 receptor in human MOLT4 cells transfected with Gqi5 assessed as inhibition of RANTES-induced calcium mobilization incubat... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50558606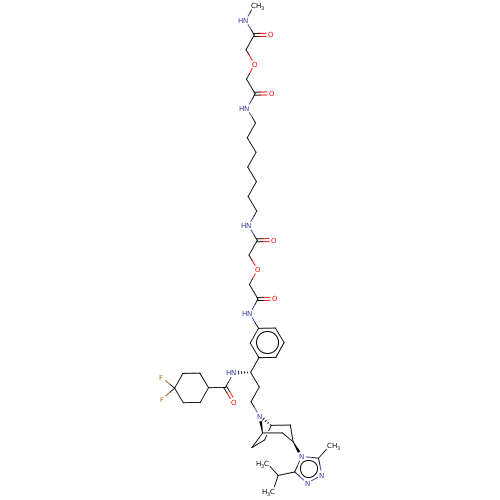 (CHEMBL4799343) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 receptor in human MOLT4 cells transfected with Gqi5 assessed as inhibition of RANTES-induced calcium mobilization incubat... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50292919 (17-Cyclopropylmethyl-3,14beta-dihydroxy-4,5r-epoxy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at MOR (unknown origin) assessed as inhibition of DAMGO-induced agonism | Bioorg Med Chem 21: 6405-13 (2013) Article DOI: 10.1016/j.bmc.2013.08.042 BindingDB Entry DOI: 10.7270/Q2QC06F7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50558607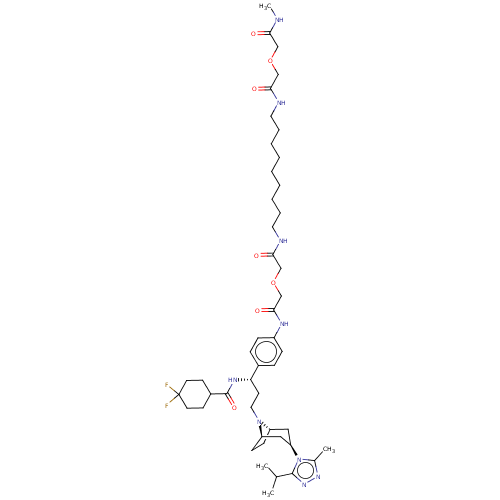 (CHEMBL4761614) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 receptor in human MOLT4 cells transfected with Gqi5 assessed as inhibition of RANTES-induced calcium mobilization incubat... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50558603 (CHEMBL4761986) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 543 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 receptor in human MOLT4 cells transfected with Gqi5 assessed as inhibition of RANTES-induced calcium mobilization incubat... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50496849 (CHEMBL3219779) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Antagonist activity at CCR5 in Gqi5 transfected human MOLT4 cells assessed as inhibition of RANTES-stimulated Ca2+ influx preincubated for 15 mins fo... | Medchemcomm 4: 847-851 (2013) Article DOI: 10.1039/c3md00080j BindingDB Entry DOI: 10.7270/Q2474DTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50558608 (CHEMBL4795179) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 receptor in human MOLT4 cells transfected with Gqi5 assessed as inhibition of RANTES-induced calcium mobilization incubat... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50558609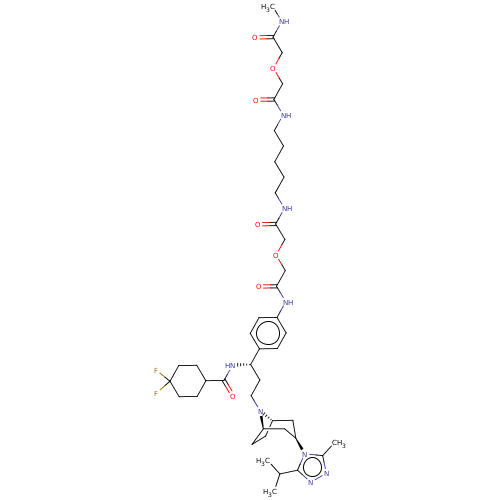 (CHEMBL4800480) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 948 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR5 receptor in human MOLT4 cells transfected with Gqi5 assessed as inhibition of RANTES-induced calcium mobilization incubat... | Citation and Details Article DOI: 10.1016/j.bmc.2016.09.059 BindingDB Entry DOI: 10.7270/Q2HD80CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 146 total ) | Next | Last >> |