

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50093526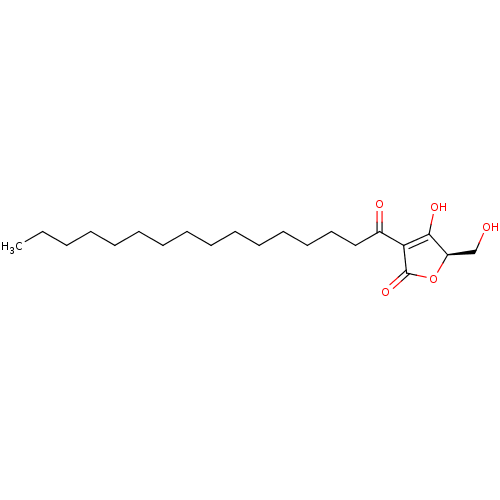 (CHEMBL426373 | RK-682) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268304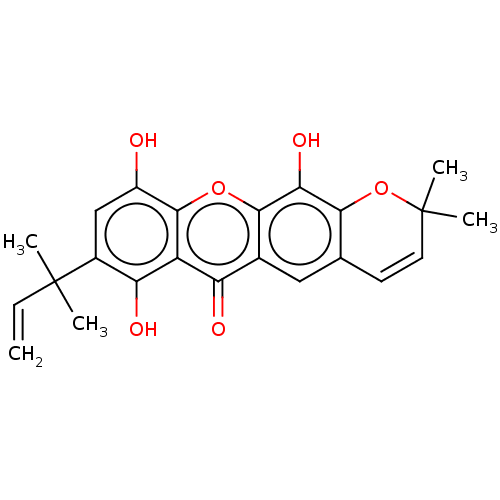 (CHEMBL4086905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268289 (CHEMBL4089362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268298 (CHEMBL4059520) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268296 (CHEMBL4069603) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268302 (CHEMBL4104006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268303 (CHEMBL4086154) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268297 (CHEMBL4068177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268301 (CHEMBL4093732) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268300 (CHEMBL4073032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268299 (CHEMBL4081488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50268290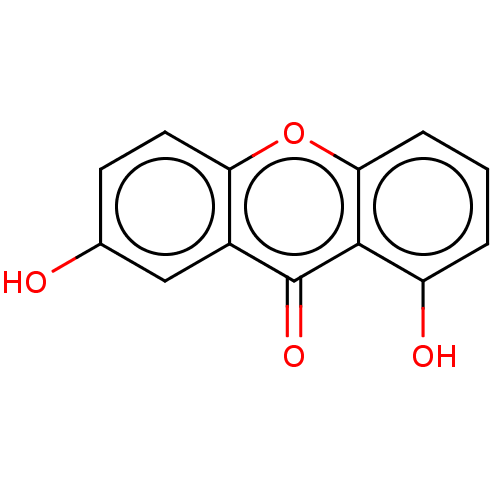 (1,7-Dihydroxyxanthone | CHEBI:4946 | CHEMBL389166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Natural Product and Medicinal Chemistry Lab, Faculty of Chemistry, VNUHCM-University of Science, 227 Nguyen Van Cu, Ho Chi Minh City, Viet Nam. Curated by ChEMBL | Assay Description Inhibition of human recombinant PTP1B pre-incubated for 10 mins before pnitrophenyl phosphate substrate addition and measured every 30 secs for 10 mi... | Bioorg Med Chem Lett 27: 3301-3304 (2017) Article DOI: 10.1016/j.bmcl.2017.06.021 BindingDB Entry DOI: 10.7270/Q20Z75QD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||