 Found 832 hits with Last Name = 'ho' and Initial = 'cs'
Found 832 hits with Last Name = 'ho' and Initial = 'cs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Pyruvate decarboxylase
(Zymomonas mobilis subsp. mobilis (strain ATCC 3182...) | BDBM50589544
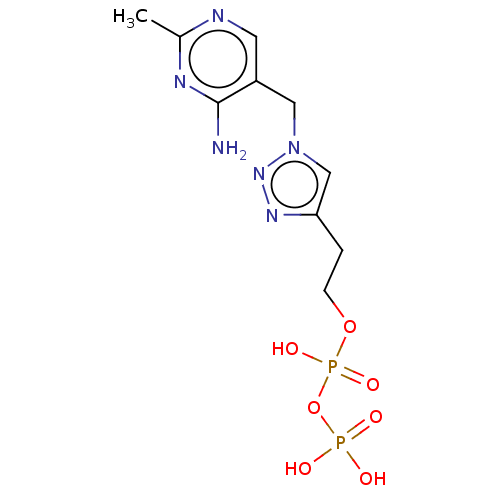
(CHEMBL3559521)Show SMILES Cc1ncc(Cn2cc(CCOP(O)(=O)OP(O)(O)=O)nn2)c(N)n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00085g
BindingDB Entry DOI: 10.7270/Q2M90DMB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM190270
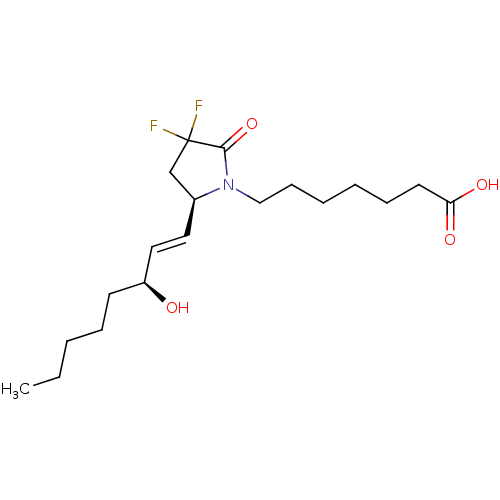
(US9180116, 9C)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CC(F)(F)C(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C19H31F2NO4/c1-2-3-6-9-16(23)12-11-15-14-19(20,21)18(26)22(15)13-8-5-4-7-10-17(24)25/h11-12,15-16,23H,2-10,13-14H2,1H3,(H,24,25)/b12-11+/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM190268

(US9180116, 2C)Show SMILES CCC#CC[C@H](C)[C@H](O)\C=C\[C@H]1CC(F)(F)C(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C21H31F2NO4/c1-3-4-7-10-16(2)18(25)13-12-17-15-21(22,23)20(28)24(17)14-9-6-5-8-11-19(26)27/h12-13,16-18,25H,3,5-6,8-11,14-15H2,1-2H3,(H,26,27)/b13-12+/t16-,17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769
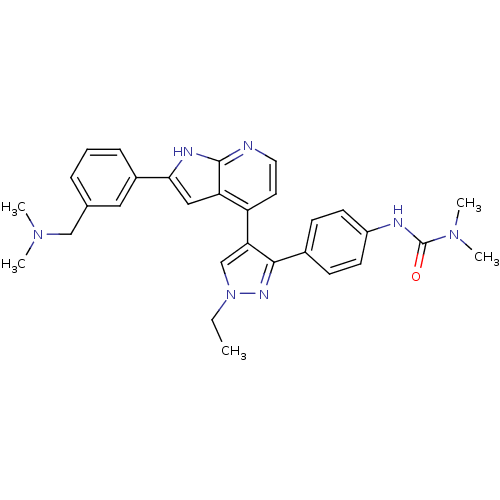
(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora B ATP binding site by rapid dilution method |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50101858
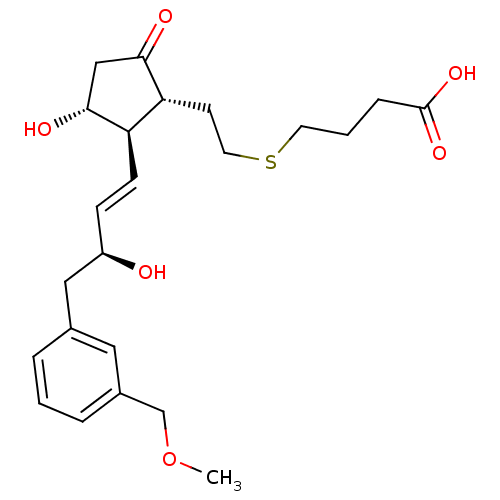
(4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...)Show SMILES COCc1cccc(C[C@H](O)\C=C\[C@H]2[C@H](O)CC(=O)[C@@H]2CCSCCCC(O)=O)c1 Show InChI InChI=1S/C23H32O6S/c1-29-15-17-5-2-4-16(12-17)13-18(24)7-8-19-20(22(26)14-21(19)25)9-11-30-10-3-6-23(27)28/h2,4-5,7-8,12,18-21,24-25H,3,6,9-11,13-15H2,1H3,(H,27,28)/b8-7+/t18-,19-,20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase C
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50142481
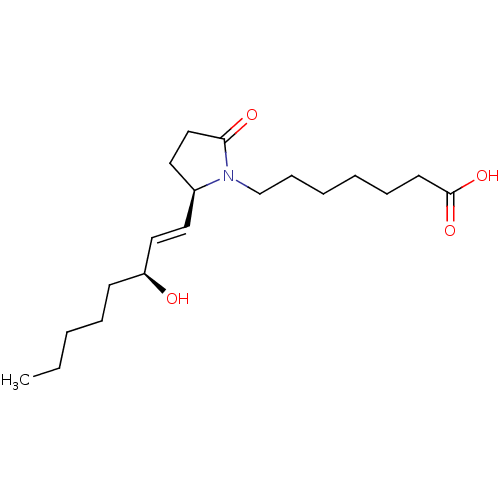
(7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C19H33NO4/c1-2-3-6-9-17(21)13-11-16-12-14-18(22)20(16)15-8-5-4-7-10-19(23)24/h11,13,16-17,21H,2-10,12,14-15H2,1H3,(H,23,24)/b13-11+/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora B ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50521600
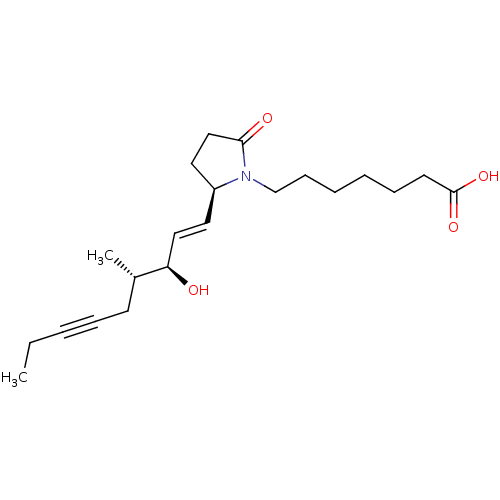
(CHEMBL4558749)Show SMILES CCC#CC[C@H](C)[C@H](O)\C=C\[C@H]1CCC(=O)N1CCCCCCC(O)=O |r| Show InChI InChI=1S/C21H33NO4/c1-3-4-7-10-17(2)19(23)14-12-18-13-15-20(24)22(18)16-9-6-5-8-11-21(25)26/h12,14,17-19,23H,3,5-6,8-11,13,15-16H2,1-2H3,(H,25,26)/b14-12+/t17-,18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM13534

(CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...)Show SMILES CN1CCN(CC1)c1cc(Nc2cc(C)n[nH]2)nc(Sc2ccc(NC(=O)C3CC3)cc2)n1 Show InChI InChI=1S/C23H28N8OS/c1-15-13-20(29-28-15)25-19-14-21(31-11-9-30(2)10-12-31)27-23(26-19)33-18-7-5-17(6-8-18)24-22(32)16-3-4-16/h5-8,13-14,16H,3-4,9-12H2,1-2H3,(H,24,32)(H2,25,26,27,28,29) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of Aurora C ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG/His-tagged HDAC1 (1 to 482 residues) expressed in sf9 cells using acetyl-Lys(Ac)-AMC as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human C-terminal His-tagged HDAC3 (1 to 428 residues)/human N-terminal GST-tagged NcoR2 (395 to 489 residues) expressed in sf9 cells us... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50446481
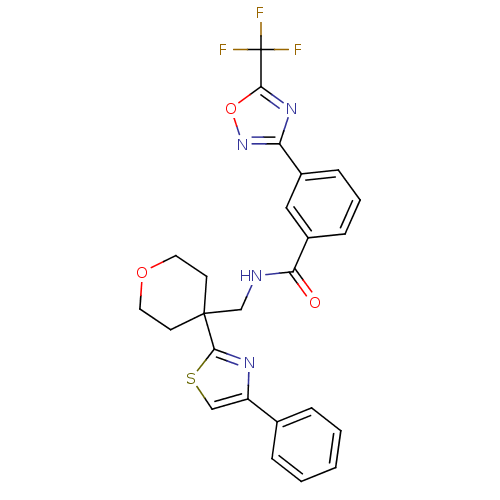
(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC9 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial
(Sus scrofa) | BDBM50615069
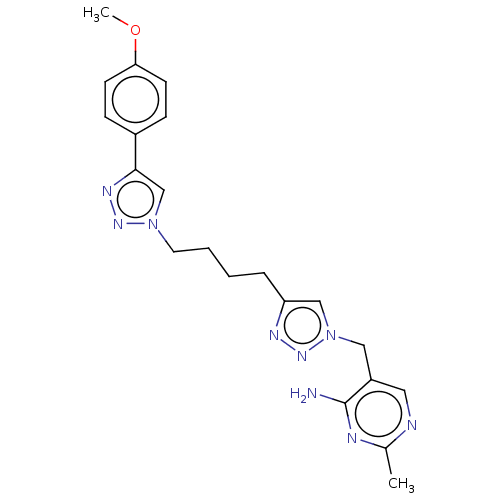
(CHEMBL5271689)Show SMILES COc1ccc(cc1)-c1cn(CCCCc2cn(Cc3cnc(C)nc3N)nn2)nn1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC7 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial
(Sus scrofa) | BDBM50615071

(CHEMBL5290932) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Pyruvate dehydrogenase E1 component subunit alpha, somatic form, mitochondrial
(Sus scrofa) | BDBM50615070

(CHEMBL5268763) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC5 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC4 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM207628
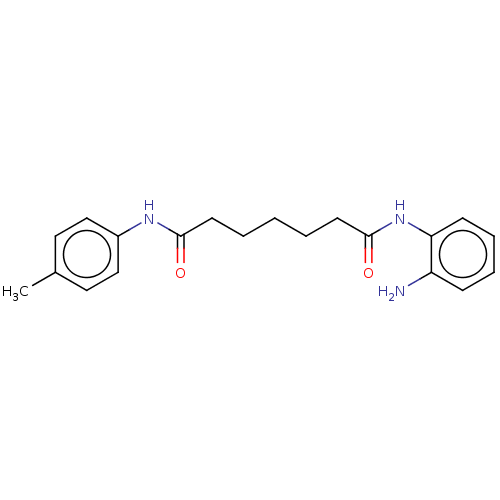
(US9265734, RGFA8 | US9796664, Compound RGFA8)Show InChI InChI=1S/C20H25N3O2/c1-15-11-13-16(14-12-15)22-19(24)9-3-2-4-10-20(25)23-18-8-6-5-7-17(18)21/h5-8,11-14H,2-4,9-10,21H2,1H3,(H,22,24)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human C-terminal FLAG/His-tagged HDAC1 (1 to 482 residues) expressed in sf9 cells using acetyl-Lys(Ac)-AMC as substrate |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM190268

(US9180116, 2C)Show SMILES CCC#CC[C@H](C)[C@H](O)\C=C\[C@H]1CC(F)(F)C(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C21H31F2NO4/c1-3-4-7-10-16(2)18(25)13-12-17-15-21(22,23)20(28)24(17)14-9-6-5-8-11-19(26)27/h12-13,16-18,25H,3,5-6,8-11,14-15H2,1-2H3,(H,26,27)/b13-12+/t16-,17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM190270

(US9180116, 9C)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CC(F)(F)C(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C19H31F2NO4/c1-2-3-6-9-16(23)12-11-15-14-19(20,21)18(26)22(15)13-8-5-4-7-10-17(24)25/h11-12,15-16,23H,2-10,13-14H2,1H3,(H,24,25)/b12-11+/t15-,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM190270

(US9180116, 9C)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CC(F)(F)C(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C19H31F2NO4/c1-2-3-6-9-16(23)12-11-15-14-19(20,21)18(26)22(15)13-8-5-4-7-10-17(24)25/h11-12,15-16,23H,2-10,13-14H2,1H3,(H,24,25)/b12-11+/t15-,16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50565137
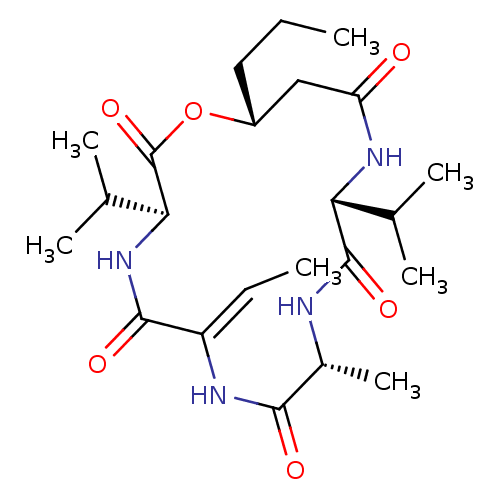
(CHEMBL4800126)Show SMILES CCC[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N[C@H](C)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC2 expressed in Sf9 cells using Ac-Leu-Gly-Lys(Ac)-AMC as substrate measured every 30 secs for 60 mins by fluorescence-based a... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Competitive inhibition of human Aurora A ATP binding site |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50315769

(3-(4-(4-(2-(3-((dimethylamino)methyl)phenyl)-1H-py...)Show SMILES CCn1cc(c(n1)-c1ccc(NC(=O)N(C)C)cc1)-c1ccnc2[nH]c(cc12)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C30H33N7O/c1-6-37-19-26(28(34-37)21-10-12-23(13-11-21)32-30(38)36(4)5)24-14-15-31-29-25(24)17-27(33-29)22-9-7-8-20(16-22)18-35(2)3/h7-17,19H,6,18H2,1-5H3,(H,31,33)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 492 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A |
J Med Chem 53: 3973-4001 (2010)
Article DOI: 10.1021/jm901870q
BindingDB Entry DOI: 10.7270/Q27082CK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50565137

(CHEMBL4800126)Show SMILES CCC[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N[C@H](C)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) using Ac-Leu-Gly-Lys(Ac)-AMC as substrate measured every 30 secs for 60 mins by fluorescence-based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50565137

(CHEMBL4800126)Show SMILES CCC[C@@H]1CC(=O)N[C@H](C(C)C)C(=O)N[C@H](C)C(=O)N\C(=C/C)C(=O)N[C@@H](C(C)C)C(=O)O1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human C-terminal His-tagged HDAC3 (1 to 428 residues)/human N-terminal GST-tagged NcoR2 (395 to 489 residues) expressed in sf9 cells us... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50521600

(CHEMBL4558749)Show SMILES CCC#CC[C@H](C)[C@H](O)\C=C\[C@H]1CCC(=O)N1CCCCCCC(O)=O |r| Show InChI InChI=1S/C21H33NO4/c1-3-4-7-10-17(2)19(23)14-12-18-13-15-20(24)22(18)16-9-6-5-8-11-21(25)26/h12,14,17-19,23H,3,5-6,8-11,13,15-16H2,1-2H3,(H,25,26)/b14-12+/t17-,18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50142481

(7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C19H33NO4/c1-2-3-6-9-17(21)13-11-16-12-14-18(22)20(16)15-8-5-4-7-10-19(23)24/h11,13,16-17,21H,2-10,12,14-15H2,1H3,(H,23,24)/b13-11+/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50142481

(7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C19H33NO4/c1-2-3-6-9-17(21)13-11-16-12-14-18(22)20(16)15-8-5-4-7-10-19(23)24/h11,13,16-17,21H,2-10,12,14-15H2,1H3,(H,23,24)/b13-11+/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using p53 residues 379-382 (RHKKAc) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC10 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC8 using p53 residues 379-382 (RHKAcKAc) as substrate as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM190268

(US9180116, 2C)Show SMILES CCC#CC[C@H](C)[C@H](O)\C=C\[C@H]1CC(F)(F)C(=O)N1CCCCCCC(O)=O Show InChI InChI=1S/C21H31F2NO4/c1-3-4-7-10-16(2)18(25)13-12-17-15-21(22,23)20(28)24(17)14-9-6-5-8-11-19(26)27/h12-13,16-18,25H,3,5-6,8-11,14-15H2,1-2H3,(H,26,27)/b13-12+/t16-,17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50300128

(1-(3-(1H-tetrazol-5-yl)propyl)-4-(benzhydryloxy)pi...)Show SMILES C(CN1CCC(CC1)OC(c1ccccc1)c1ccccc1)Cc1nnn[nH]1 Show InChI InChI=1S/C22H27N5O/c1-3-8-18(9-4-1)22(19-10-5-2-6-11-19)28-20-13-16-27(17-14-20)15-7-12-21-23-25-26-24-21/h1-6,8-11,20,22H,7,12-17H2,(H,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human HPGDS using PGH2 as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127759
BindingDB Entry DOI: 10.7270/Q2VD7346 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50521600

(CHEMBL4558749)Show SMILES CCC#CC[C@H](C)[C@H](O)\C=C\[C@H]1CCC(=O)N1CCCCCCC(O)=O |r| Show InChI InChI=1S/C21H33NO4/c1-3-4-7-10-17(2)19(23)14-12-18-13-15-20(24)22(18)16-9-6-5-8-11-21(25)26/h12,14,17-19,23H,3,5-6,8-11,13,15-16H2,1-2H3,(H,25,26)/b14-12+/t17-,18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... |
J Med Chem 62: 4731-4741 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00336
BindingDB Entry DOI: 10.7270/Q22R3W32 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC2 using p53 (379 to 382 residues) (RHKKAc) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC11 using (Boc-Lys(trifluoroacetyl)-AMC) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC1 using p53 (379 to 382 residues) (RHKKAc) as substrate measured after 2 hrs by cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50446481

(CHEMBL3110004 | US10011611, TMP269 | US10722597, C...)Show SMILES FC(F)(F)c1nc(no1)-c1cccc(c1)C(=O)NCC1(CCOCC1)c1nc(cs1)-c1ccccc1 Show InChI InChI=1S/C25H21F3N4O3S/c26-25(27,28)22-31-20(32-35-22)17-7-4-8-18(13-17)21(33)29-15-24(9-11-34-12-10-24)23-30-19(14-36-23)16-5-2-1-3-6-16/h1-8,13-14H,9-12,15H2,(H,29,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| >4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC3/NcoR2 using p53 residues 379-382 (RHKKAc) as substrate measured after 2 hrs by Cheng-Prusoff analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Pyruvate decarboxylase
(Zymomonas mobilis subsp. mobilis (strain ATCC 3182...) | BDBM50589543
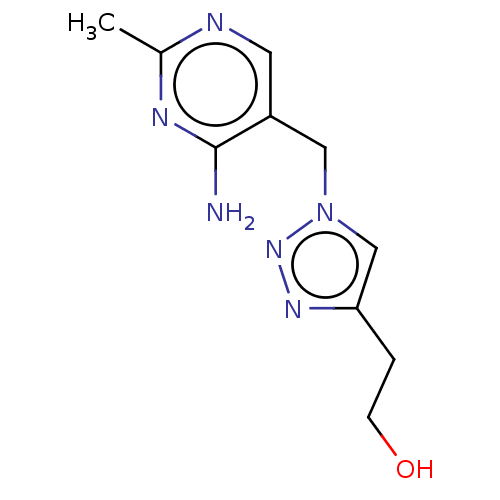
(CHEMBL4277786) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00085g
BindingDB Entry DOI: 10.7270/Q2M90DMB |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50105327
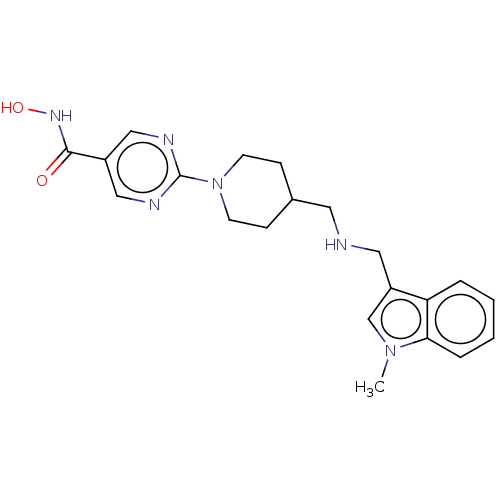
(JNJ-26481585 | Quisinostat)Show SMILES Cn1cc(CNCC2CCN(CC2)c2ncc(cn2)C(=O)NO)c2ccccc12 Show InChI InChI=1S/C21H26N6O2/c1-26-14-17(18-4-2-3-5-19(18)26)11-22-10-15-6-8-27(9-7-15)21-23-12-16(13-24-21)20(28)25-29/h2-5,12-15,22,29H,6-11H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC1 expressed in baculovirus infected sf9 cells using p53 residues 379-382 (RHKKAc) as substrate by fluorescence-based assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |
Tyrosine--tRNA ligase
(Staphylococcus aureus) | BDBM18131

(SB-243545 | butyl (2S)-2-[(2S)-2-amino-3-(4-hydrox...)Show SMILES [H][C@]1([C@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)OCCCC)[C@H](O)[C@](O)(CO)[C@@H](O)CN1O |r| Show InChI InChI=1S/C21H33N3O9/c1-2-3-8-33-20(30)16(17-18(28)21(31,11-25)15(27)10-24(17)32)23-19(29)14(22)9-12-4-6-13(26)7-5-12/h4-7,14-18,25-28,31-32H,2-3,8-11,22H2,1H3,(H,23,29)/t14-,15-,16-,17-,18-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.9 | 37 |
GSK
| Assay Description
The concentration of inhibitor which results in 50% inhibition (IC50) of enzyme activity was determined by preincubating recombinant S. aureus TyrRS ... |
Protein Sci 10: 2008-16 (2001)
Article DOI: 10.1110/ps.18001
BindingDB Entry DOI: 10.7270/Q2NG4NXT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410
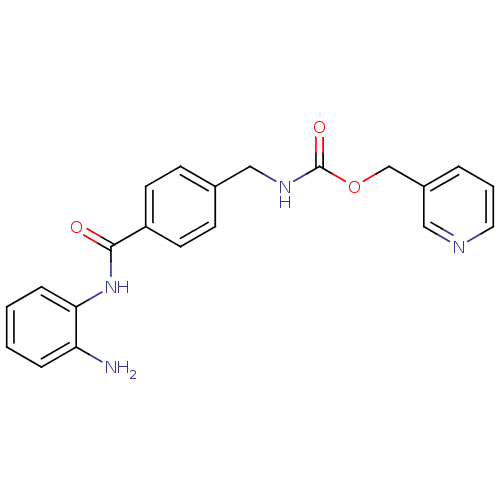
(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human HDAC1 using pan-HDAC substrate incubated for 3 hrs by fluorescence method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00830
BindingDB Entry DOI: 10.7270/Q2F76H9K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data