

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184323 (CHEMBL379040 | phosphoric acid mono-{(2R,3R,4R,5R,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184324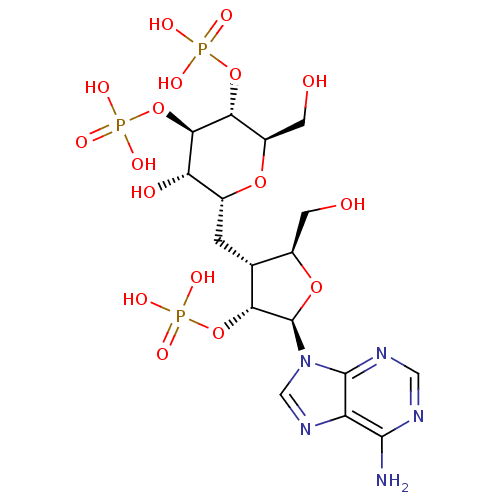 (CHEMBL383049 | phosphoric acid mono-{(2R,3R,4R,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184326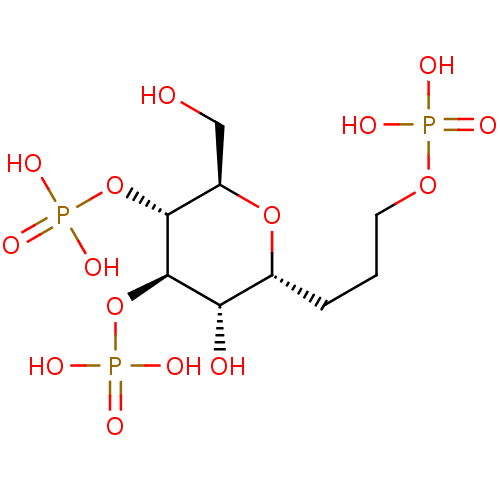 (CHEMBL380941 | phosphoric acid mono-[(2R,3R,4R,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 213 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184329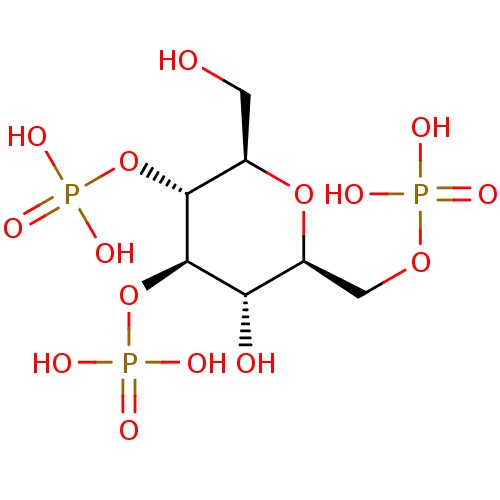 (CHEMBL383659 | phosphoric acid mono-((2R,3R,4R,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184325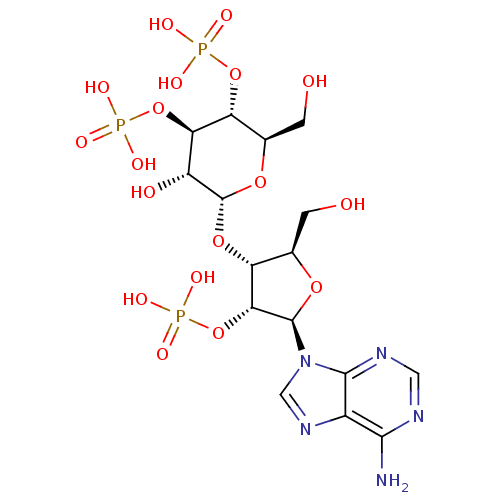 (2-[5-(6-amino-9H-9-purinyl)-2-hydroxymethyl-4-oxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184328 (CHEMBL204903 | phosphoric acid mono-[(2R,3R,4R,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50075183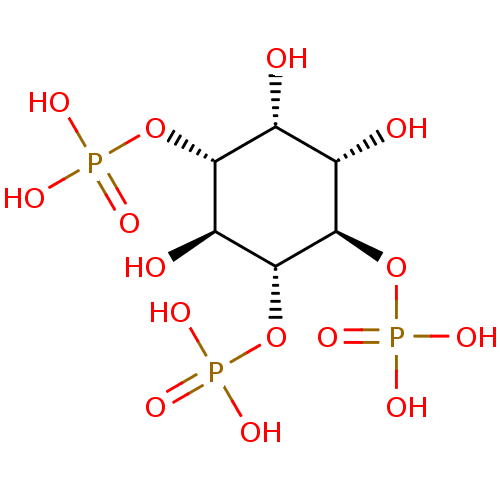 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 24.8 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184327 (CHEMBL205303 | phosphoric acid mono-[(2R,3R,4R,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.43E+3 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184331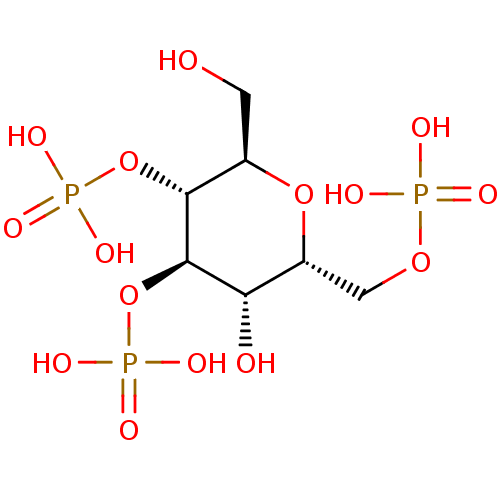 (CHEMBL204253 | phosphoric acid mono-((2R,3R,4R,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 394 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184332 (CHEMBL382185 | phosphoric acid mono-{(2R,3R,4R,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11.3 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184330 (CHEMBL205008 | phosphoric acid mono-[(2R,3R,4R,5S,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 49.2 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Agonistic potency at rat IP3 type 1 receptor expressed in chicken DT40 cells | J Med Chem 49: 1900-9 (2006) Article DOI: 10.1021/jm051039n BindingDB Entry DOI: 10.7270/Q2MK6CG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50075183 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184325 (2-[5-(6-amino-9H-9-purinyl)-2-hydroxymethyl-4-oxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323704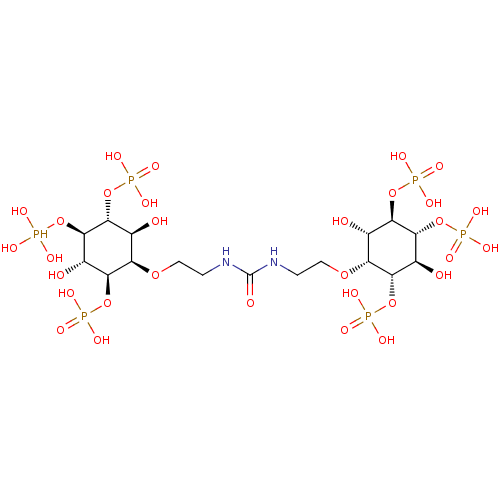 (CHEMBL1213158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323705 (CHEMBL1213159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323706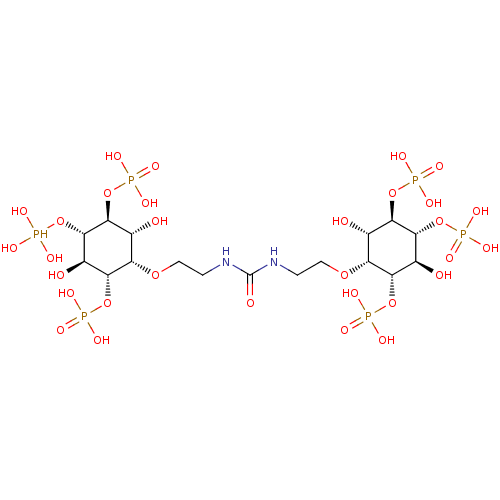 (CHEMBL1212943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323707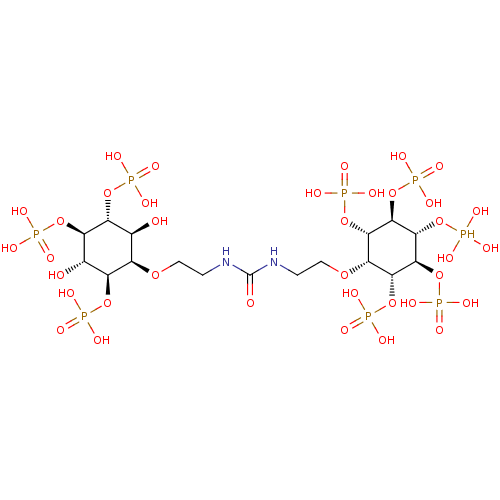 (CHEMBL1213160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323708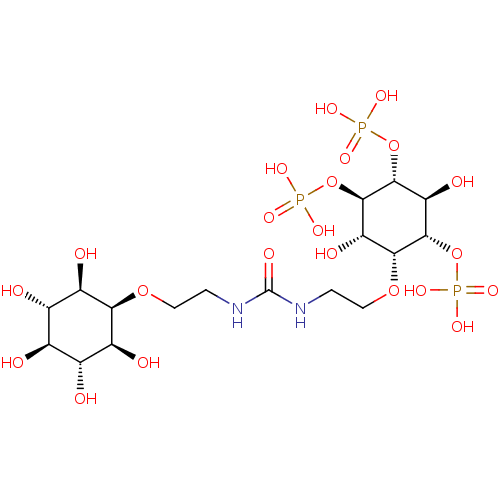 (CHEMBL1213161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323709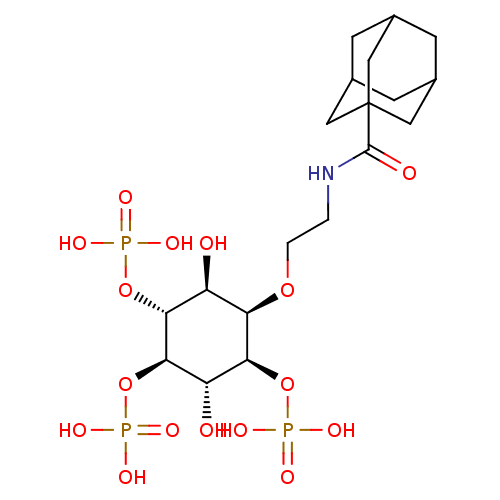 (CHEMBL1213163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323710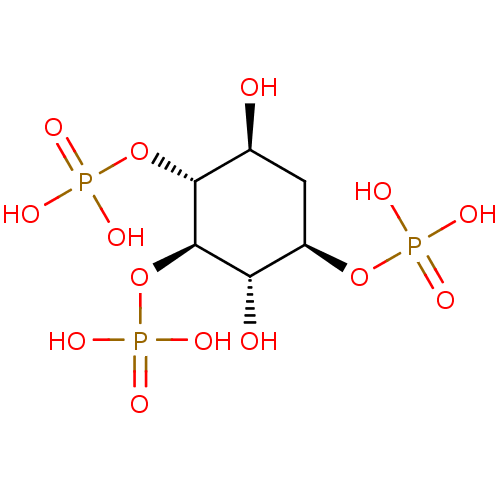 ((1R,2R,3S,4R,6S)-3,6-dihydroxycyclohexane-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323711 ((1R,2R,3S,4S,5R,6S)-5-(2-aminoethoxy)-3,6-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Agonist activity at rat recombinant IP3R1 expressed in chicken DT40 cells assessed as calcium release from intracellular stores | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50075183 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 12.5 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50184325 (2-[5-(6-amino-9H-9-purinyl)-2-hydroxymethyl-4-oxyp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323704 (CHEMBL1213158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323705 (CHEMBL1213159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323706 (CHEMBL1212943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323707 (CHEMBL1213160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323708 (CHEMBL1213161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.82 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323709 (CHEMBL1213163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 7.62 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323710 ((1R,2R,3S,4R,6S)-3,6-dihydroxycyclohexane-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 17.1 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323711 ((1R,2R,3S,4S,5R,6S)-5-(2-aminoethoxy)-3,6-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 43.6 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from full-length rat recombinant IP3R1 expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50075183 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 2.32 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323704 (CHEMBL1213158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323705 (CHEMBL1213159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323706 (CHEMBL1212943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323707 (CHEMBL1213160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323708 (CHEMBL1213161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.81 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323709 (CHEMBL1213163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323710 ((1R,2R,3S,4R,6S)-3,6-dihydroxycyclohexane-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.01 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323711 ((1R,2R,3S,4S,5R,6S)-5-(2-aminoethoxy)-3,6-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant N-terminal IP3R1 (1-604) expressed in chicken DT40 cells by equilibrium competitive binding assay | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50075183 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323704 (CHEMBL1213158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323705 (CHEMBL1213159) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323706 (CHEMBL1212943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323707 (CHEMBL1213160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323708 (CHEMBL1213161) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323709 (CHEMBL1213163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323710 ((1R,2R,3S,4R,6S)-3,6-dihydroxycyclohexane-1,2,4-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50323711 ((1R,2R,3S,4S,5R,6S)-5-(2-aminoethoxy)-3,6-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Displacement of [3H]IP3 from rat recombinant IP3R1 binding core residue (224-604) expressed in chicken DT40 cells by equilibrium competitive binding ... | Nat Chem Biol 5: 631-9 (2009) Article DOI: 10.1038/nchembio.195 BindingDB Entry DOI: 10.7270/Q2C53M27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol 1,4,5-trisphosphate receptor type 1 (Rattus norvegicus) | BDBM50194703 (5'-Deoxy-5'-phenyladenophostin A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
Hokkaido University Curated by ChEMBL | Assay Description Activity at rat IP3 type 1 receptor expressed in DT40 cell assessed as calcium ion mobilization | J Med Chem 49: 5750-8 (2006) Article DOI: 10.1021/jm060310d BindingDB Entry DOI: 10.7270/Q2BK1D4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 107 total ) | Next | Last >> |