

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50299206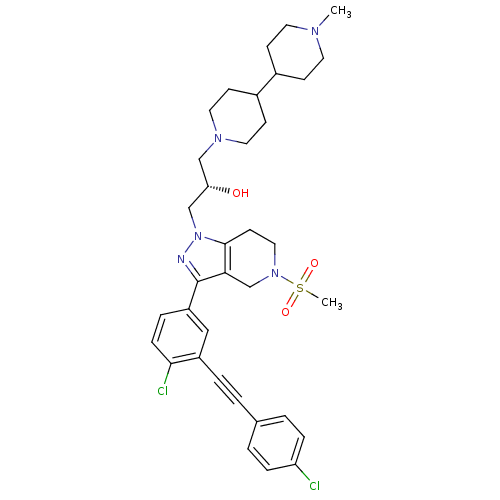 ((S)-1-(3-(4-chloro-3-((4-chlorophenyl)ethynyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299205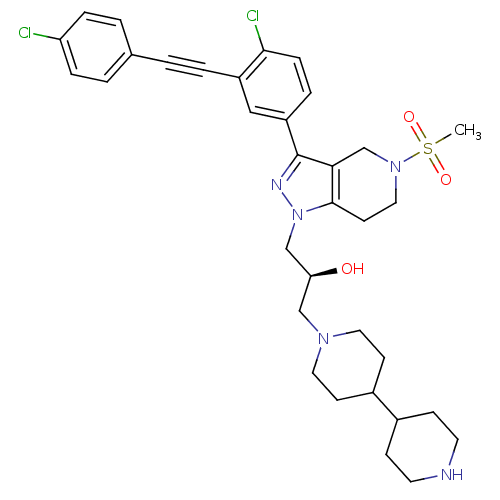 ((S)-1-(4,4'-bipiperidin-1-yl)-3-(3-(4-chloro-3-((4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299203 ((S)-tert-butyl 1'-(3-(3-(4-chloro-3-((4-chlorophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299186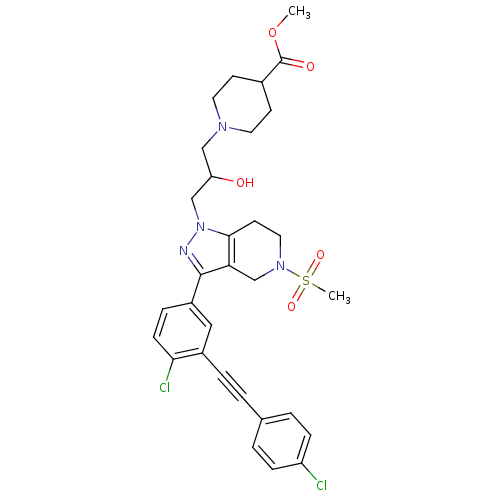 (CHEMBL573418 | methyl 1-(3-(3-(4-chloro-3-((4-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299204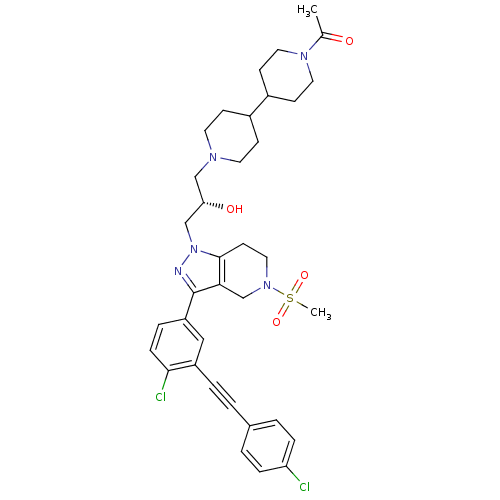 ((S)-1-(1'-(3-(3-(4-chloro-3-((4-chlorophenyl)ethyn...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314176 (4-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314168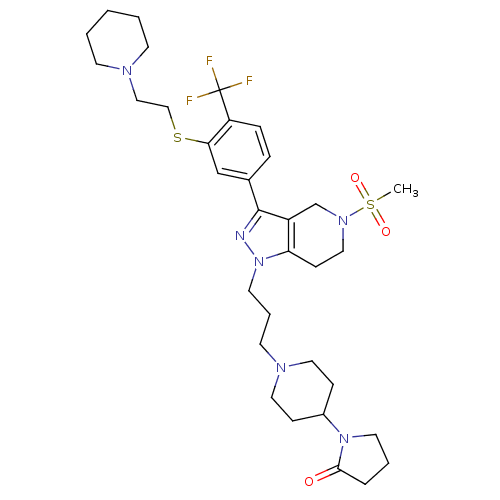 (1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50321626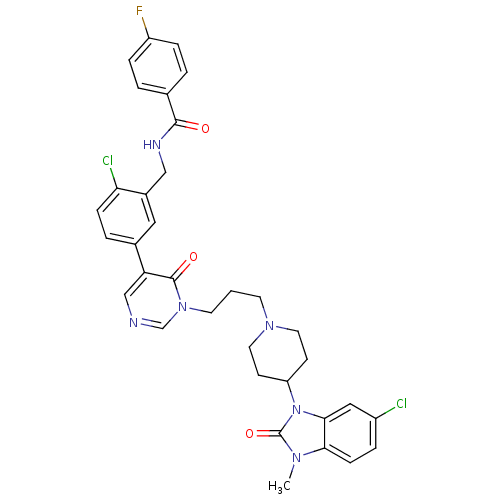 (CHEMBL1171504 | N-(2-chloro-5-(1-(3-(4-(6-chloro-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 4060-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.086 BindingDB Entry DOI: 10.7270/Q20Z747Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314171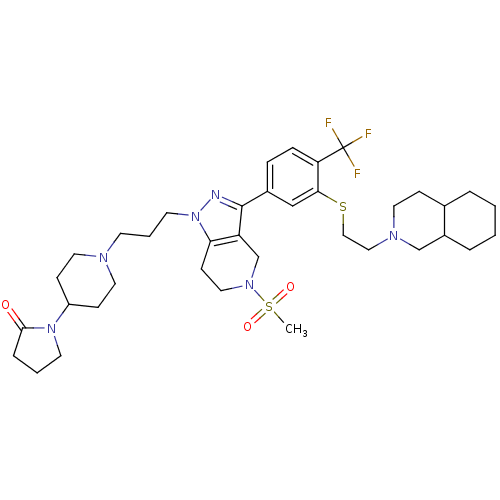 (1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(octahydroisoq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50321625 (CHEMBL1171503 | CatS_4 | N-(2-chloro-5-(2-(3-(4-(6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 4060-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.086 BindingDB Entry DOI: 10.7270/Q20Z747Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314164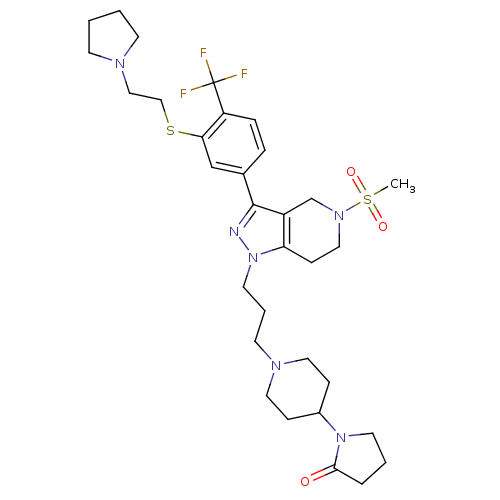 (1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(pyrrolidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299198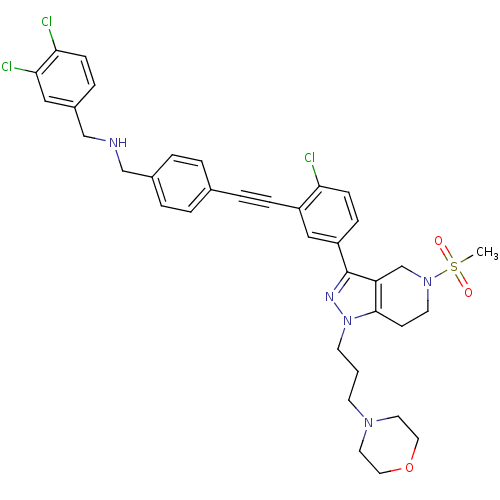 (CHEMBL573474 | N-(4-((2-chloro-5-(5-(methylsulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299185 (1-(3-(4-chloro-3-((4-chlorophenyl)ethynyl)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299201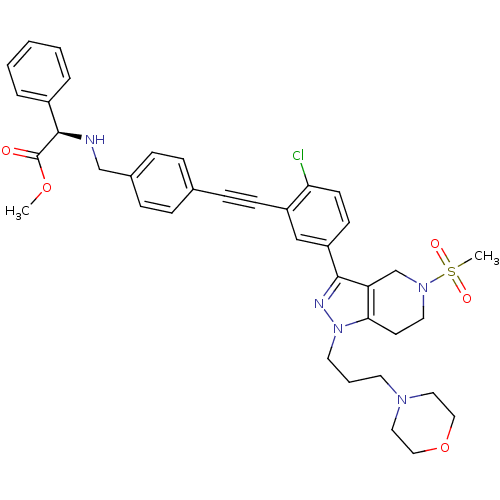 ((R)-methyl 2-(4-((2-chloro-5-(5-(methylsulfonyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299200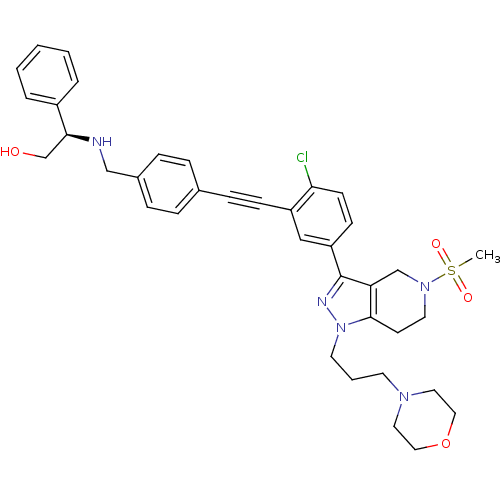 ((R)-2-(4-((2-chloro-5-(5-(methylsulfonyl)-1-(3-mor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50321627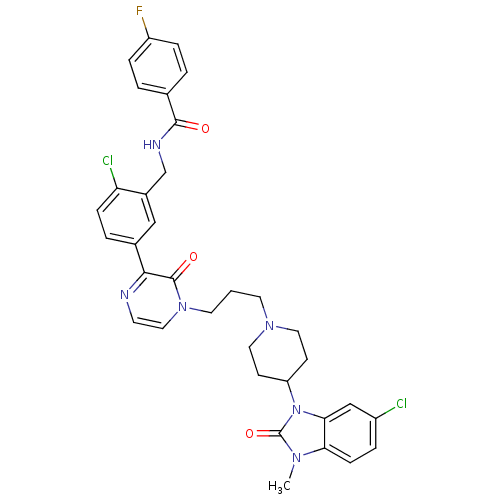 (CHEMBL1171505 | CatS_6 | N-(2-chloro-5-(4-(3-(4-(6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 4060-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.086 BindingDB Entry DOI: 10.7270/Q20Z747Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299187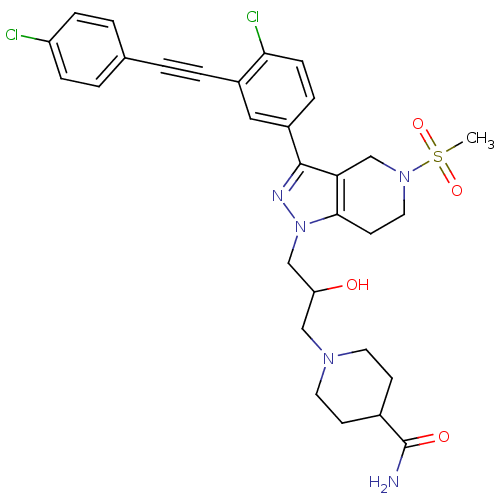 (1-(3-(3-(4-chloro-3-((4-chlorophenyl)ethynyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314170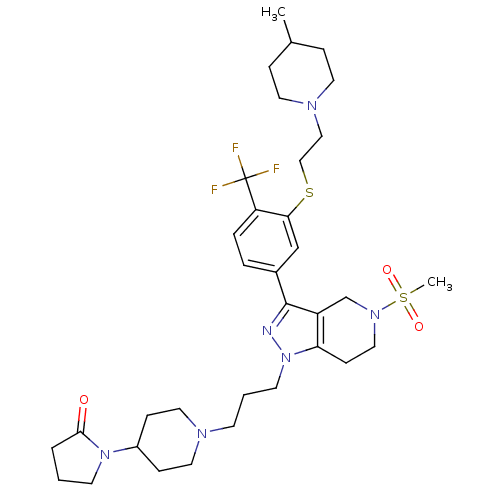 (1-(1-(3-(3-(3-(2-(4-methylpiperidin-1-yl)ethylthio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299202 ((S)-methyl 2-(4-((2-chloro-5-(5-(methylsulfonyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314181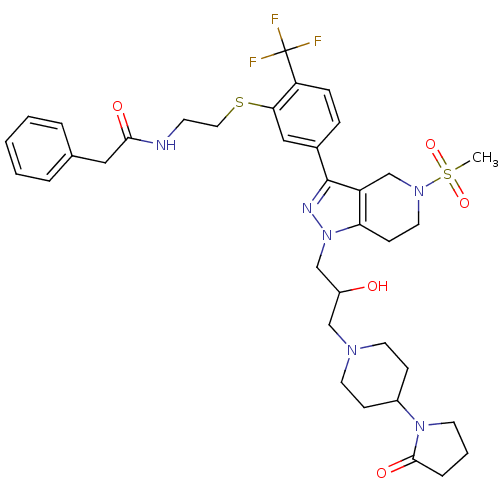 (CHEMBL1089278 | N-(2-(5-(1-(2-hydroxy-3-(4-(2-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314161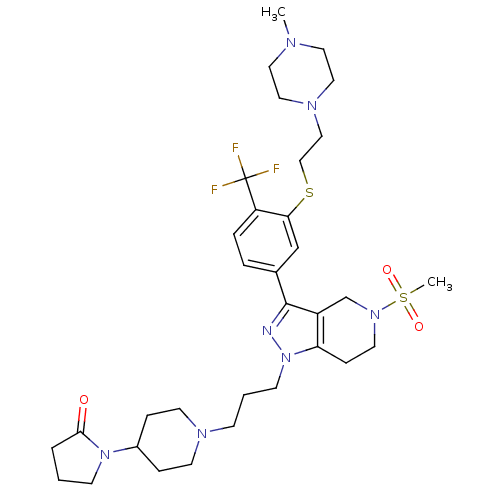 (1-(1-(3-(3-(3-(2-(4-methylpiperazin-1-yl)ethylthio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299182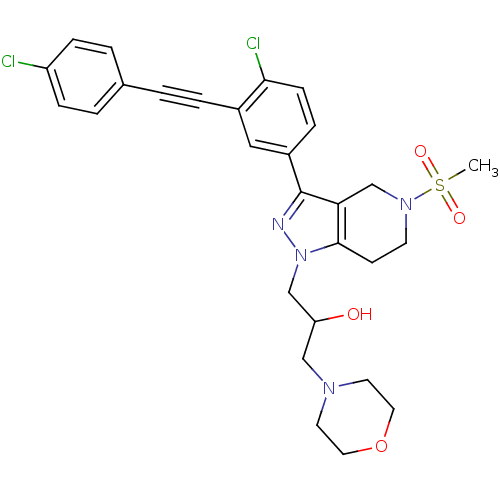 (1-(3-(4-chloro-3-((4-chlorophenyl)ethynyl)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299197 (CHEMBL573636 | N-(4-((2-chloro-5-(5-(methylsulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299183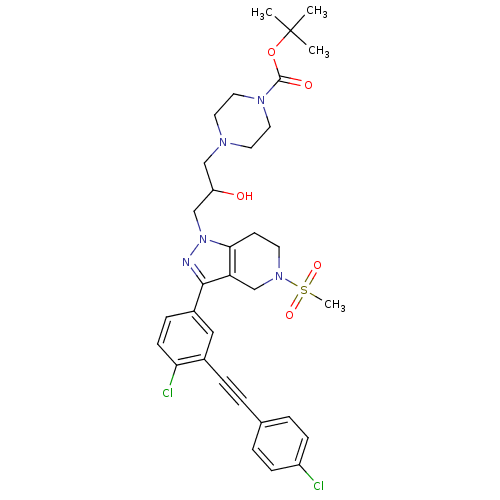 (CHEMBL573197 | tert-butyl 4-(3-(3-(4-chloro-3-((4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314169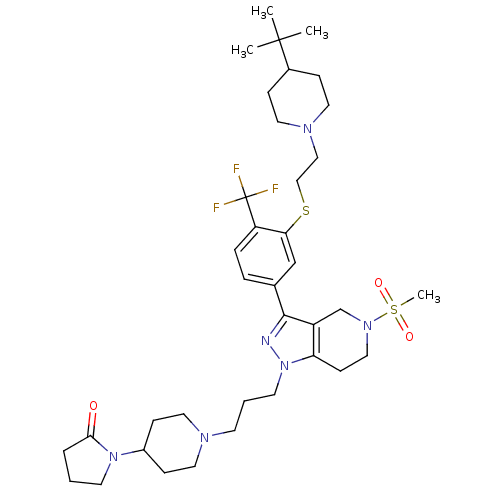 (1-(1-(3-(3-(3-(2-(4-tert-butylpiperidin-1-yl)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299189 (CHEMBL573421 | N-(4-((2-chloro-5-(5-(methylsulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314168 (1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299199 ((+/-)-N-(4-((2-chloro-5-(5-(methylsulfonyl)-1-(3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314167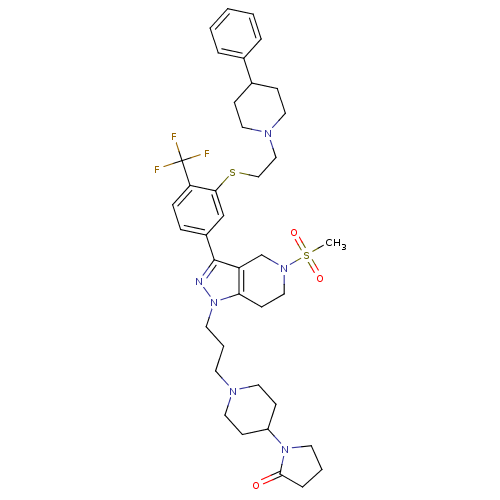 (1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(4-phenylpiper...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314157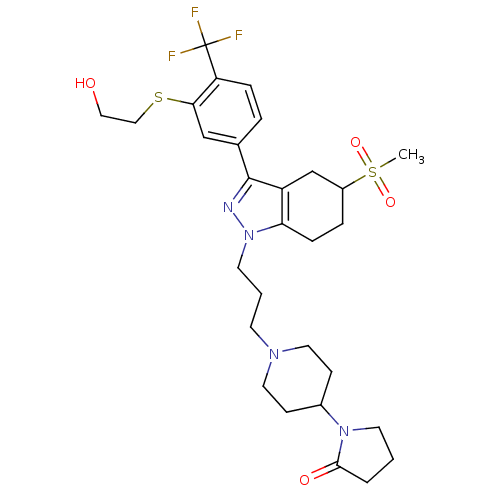 (1-(1-(3-(3-(3-(2-hydroxyethylthio)-4-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299205 ((S)-1-(4,4'-bipiperidin-1-yl)-3-(3-(4-chloro-3-((4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as invariant chain li degradation by western blotting | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314176 (4-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(piperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314156 (1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-phenoxyethylth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299196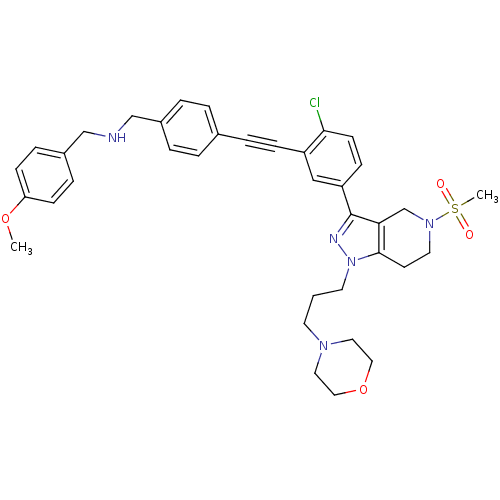 (CHEMBL573657 | N-(4-((2-chloro-5-(5-(methylsulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314166 (1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-morpholinoethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50321624 (CHEMBL1171502 | N-(2-chloro-5-(1-(3-(4-(6-chloro-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 4060-4 (2010) Article DOI: 10.1016/j.bmcl.2010.05.086 BindingDB Entry DOI: 10.7270/Q20Z747Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314173 (5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314169 (1-(1-(3-(3-(3-(2-(4-tert-butylpiperidin-1-yl)ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314165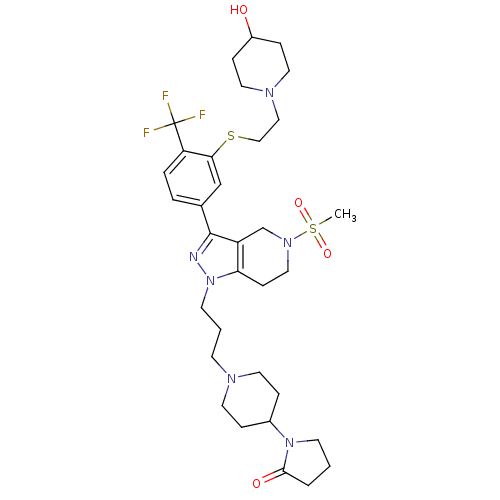 (1-(1-(3-(3-(3-(2-(4-hydroxypiperidin-1-yl)ethylthi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299195 (CHEMBL573632 | N-(4-((2-chloro-5-(5-(methylsulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314165 (1-(1-(3-(3-(3-(2-(4-hydroxypiperidin-1-yl)ethylthi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299190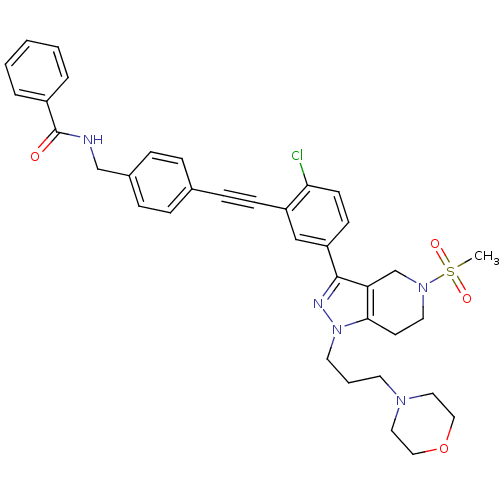 (CHEMBL573422 | N-(4-((2-chloro-5-(5-(methylsulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314179 (CHEMBL1089277 | rac-4-fluoro-N-(2-(5-(1-(2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314175 (5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells assessed as inhibition of invariant chain degradation | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314177 (CHEMBL1089275 | rac-N-(2-(5-(1-(2-hydroxy-3-(4-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314175 (5-(methylsulfonyl)-3-(3-(2-(piperidin-1-yl)ethylth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299184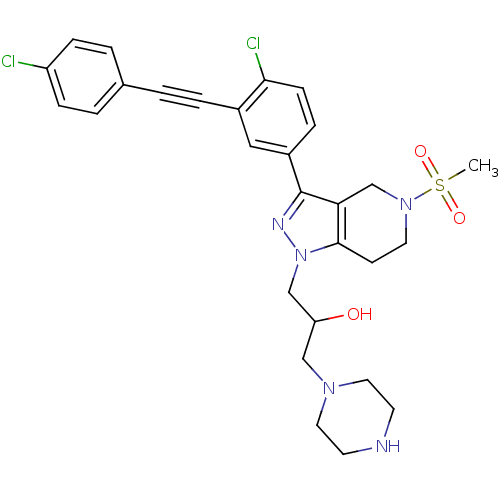 (1-(3-(4-chloro-3-((4-chlorophenyl)ethynyl)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314178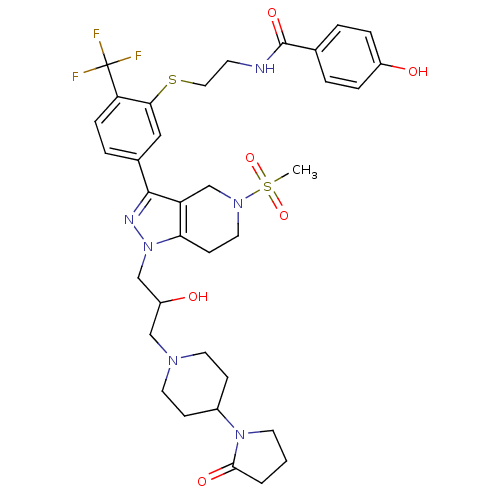 (CHEMBL1089276 | rac-4-hydroxy-N-(2-(5-(1-(2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50314164 (1-(1-(3-(5-(methylsulfonyl)-3-(3-(2-(pyrrolidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 20: 2370-4 (2010) Article DOI: 10.1016/j.bmcl.2010.01.108 BindingDB Entry DOI: 10.7270/Q2G160Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50299192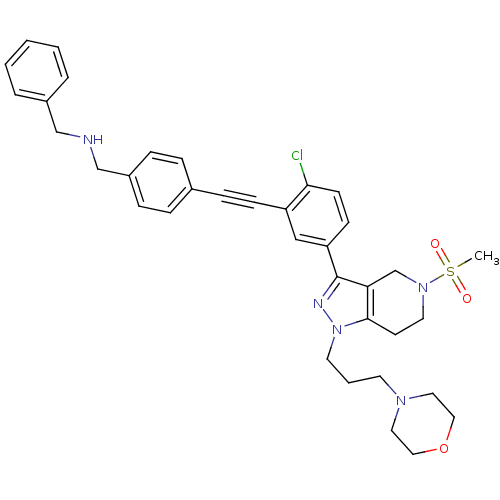 (CHEMBL573655 | N-benzyl-1-(4-((2-chloro-5-(5-(meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S expressed in baculovirus by FRET assay | Bioorg Med Chem Lett 19: 6135-9 (2009) Article DOI: 10.1016/j.bmcl.2009.09.013 BindingDB Entry DOI: 10.7270/Q2R49QTP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |