

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174389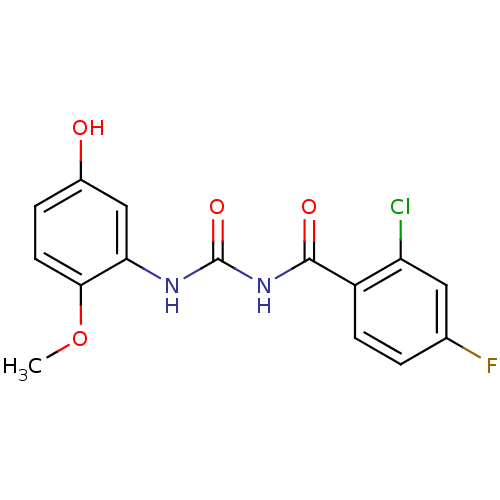 (1-(2-Chloro-4-fluoro-benzoyl)-3-(5-hydroxy-2-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 22.9 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174389 (1-(2-Chloro-4-fluoro-benzoyl)-3-(5-hydroxy-2-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50275569 (CHEMBL4130148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Displacement of [125I]GLP-1 from human GLP1R expressed in HEK293 cell membranes after 180 mins by microbeta counting method | J Med Chem 61: 5580-5593 (2018) Article DOI: 10.1021/acs.jmedchem.8b00292 BindingDB Entry DOI: 10.7270/Q2NP26XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174381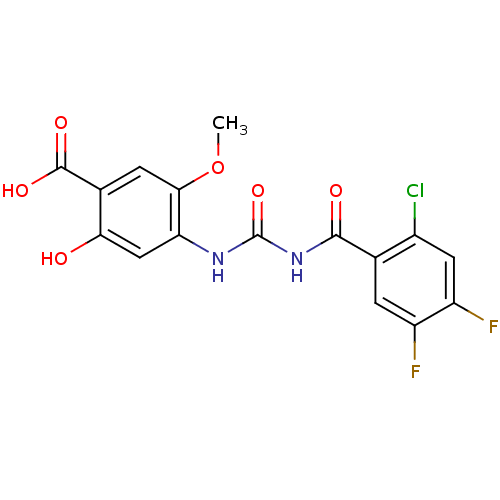 (4-[3-(2-Chloro-4,5-difluoro-benzoyl)-ureido]-2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174381 (4-[3-(2-Chloro-4,5-difluoro-benzoyl)-ureido]-2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174379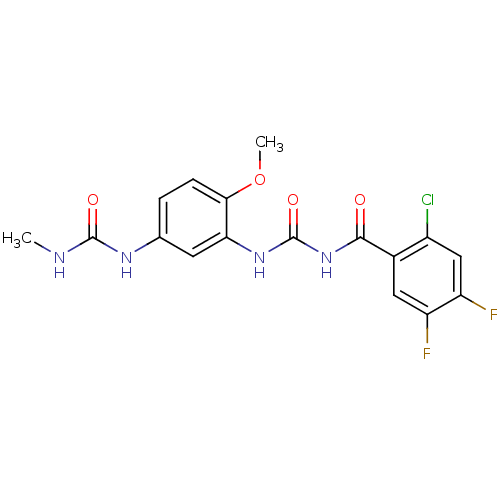 (1-{3-[3-(2-Chloro-4,5-difluoro-benzoyl)-ureido]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52.5 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174379 (1-{3-[3-(2-Chloro-4,5-difluoro-benzoyl)-ureido]-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174397 (CHEMBL370848 | N-{4-Chloro-3-[3-(2-chloro-4,5-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174375 (CHEMBL381870 | N-{3-[3-(2-Chloro-4,5-difluoro-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174397 (CHEMBL370848 | N-{4-Chloro-3-[3-(2-chloro-4,5-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60.3 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174375 (CHEMBL381870 | N-{3-[3-(2-Chloro-4,5-difluoro-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60.3 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174372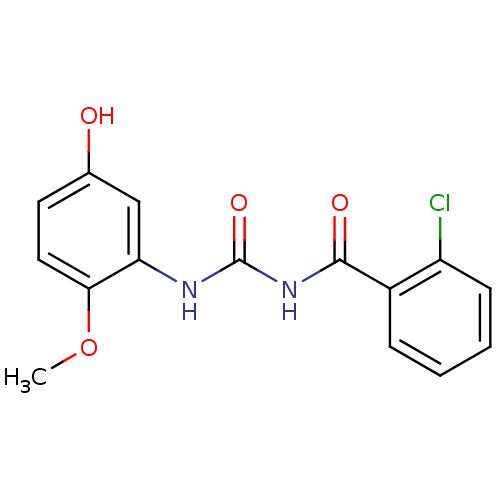 (1-(2-Chloro-benzoyl)-3-(5-hydroxy-2-methoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64.6 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174372 (1-(2-Chloro-benzoyl)-3-(5-hydroxy-2-methoxy-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174366 (1-(2-Chloro-4-fluoro-benzoyl)-3-[4-(1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174366 (1-(2-Chloro-4-fluoro-benzoyl)-3-[4-(1H-tetrazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174384 (5-Chloro-4-[3-(2-chloro-4,5-difluoro-benzoyl)-urei...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89.1 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174384 (5-Chloro-4-[3-(2-chloro-4,5-difluoro-benzoyl)-urei...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174378 (1-(2-Chloro-4-fluoro-benzoyl)-3-(3,4-dihydroxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174378 (1-(2-Chloro-4-fluoro-benzoyl)-3-(3,4-dihydroxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174405 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-3-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174373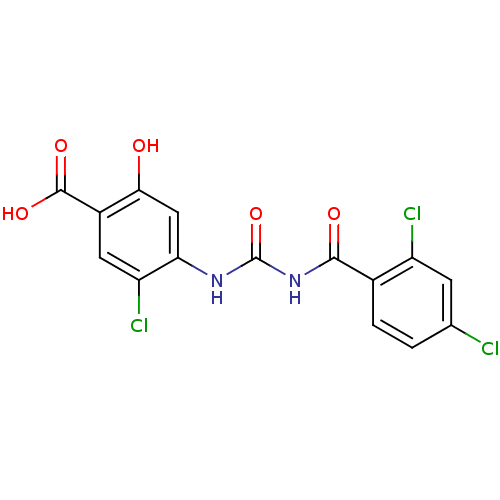 (5-Chloro-4-[3-(2,4-dichloro-benzoyl)-ureido]-2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174373 (5-Chloro-4-[3-(2,4-dichloro-benzoyl)-ureido]-2-hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174405 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-3-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 151 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174403 (1-{4-Chloro-3-[3-(2,4-dichloro-benzoyl)-ureido]-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174374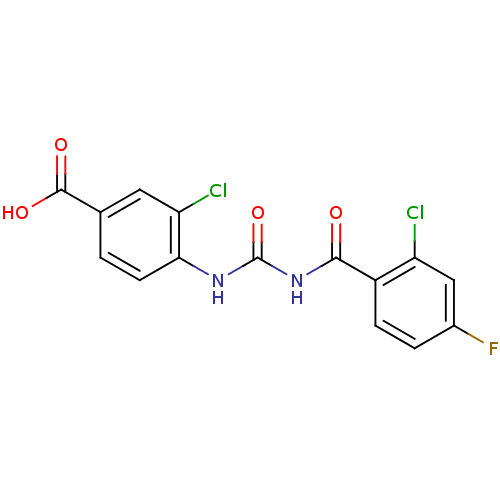 (3-Chloro-4-[3-(2-chloro-4-fluoro-benzoyl)-ureido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174362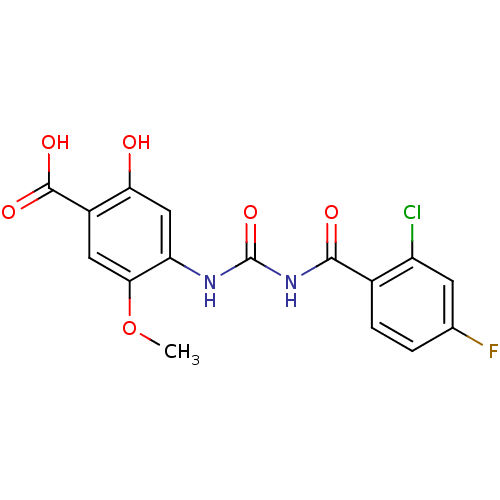 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174374 (3-Chloro-4-[3-(2-chloro-4-fluoro-benzoyl)-ureido]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174403 (1-{4-Chloro-3-[3-(2,4-dichloro-benzoyl)-ureido]-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174362 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174368 (1-(2-Chloro-4-fluoro-benzoyl)-3-[2-chloro-4-(1H-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174368 (1-(2-Chloro-4-fluoro-benzoyl)-3-[2-chloro-4-(1H-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 191 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174380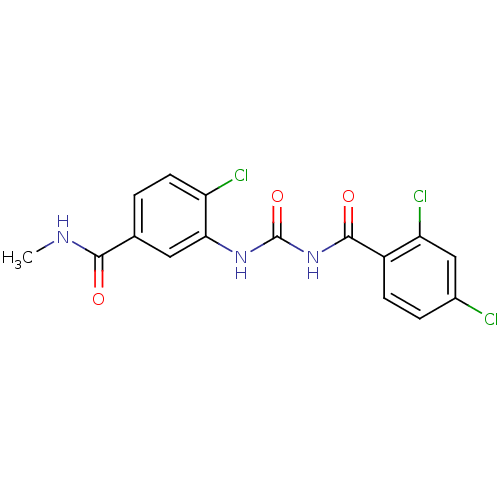 (4-Chloro-3-[3-(2,4-dichloro-benzoyl)-ureido]-N-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174380 (4-Chloro-3-[3-(2,4-dichloro-benzoyl)-ureido]-N-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174388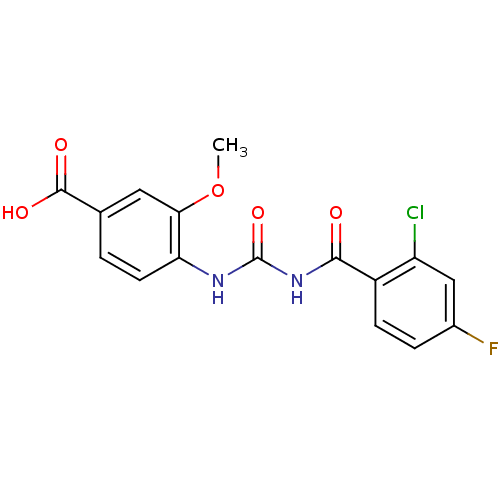 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 209 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174388 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174369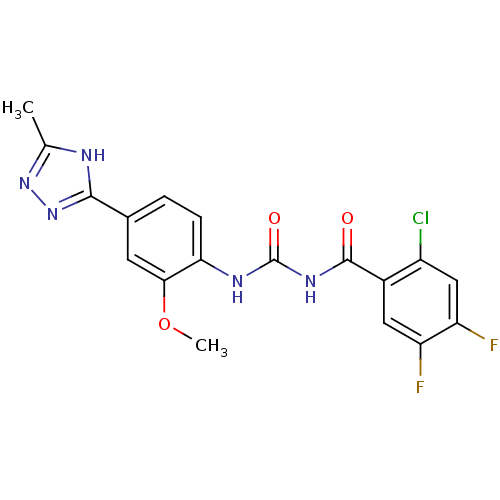 (1-(2-Chloro-4,5-difluoro-benzoyl)-3-[2-methoxy-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174369 (1-(2-Chloro-4,5-difluoro-benzoyl)-3-[2-methoxy-4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174385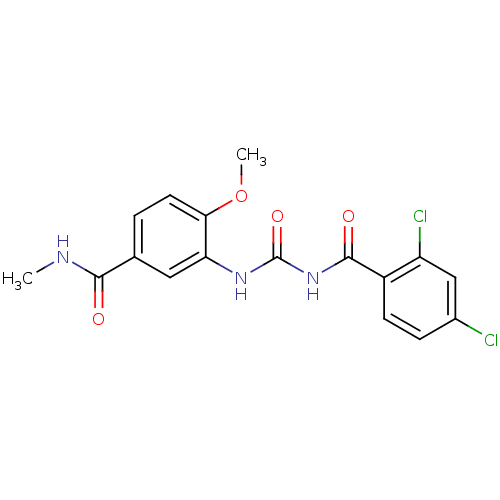 (3-[3-(2,4-Dichloro-benzoyl)-ureido]-4-methoxy-N-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174395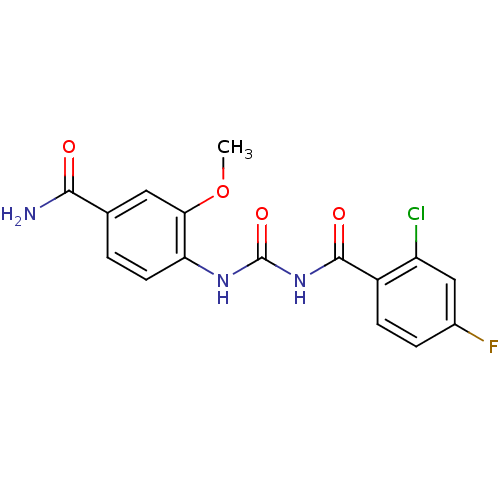 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174395 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-3-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 302 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174385 (3-[3-(2,4-Dichloro-benzoyl)-ureido]-4-methoxy-N-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 302 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174365 (4-[3-(2,4-Dichloro-benzoyl)-ureido]-3-methoxy-N-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 407 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174365 (4-[3-(2,4-Dichloro-benzoyl)-ureido]-3-methoxy-N-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174391 (3-Chloro-4-[3-(2,4-dichloro-benzoyl)-ureido]-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 417 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174391 (3-Chloro-4-[3-(2,4-dichloro-benzoyl)-ureido]-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174396 (4-[3-(2-Chloro-4,5-difluoro-benzoyl)-ureido]-3-(2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 447 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174396 (4-[3-(2-Chloro-4,5-difluoro-benzoyl)-ureido]-3-(2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174404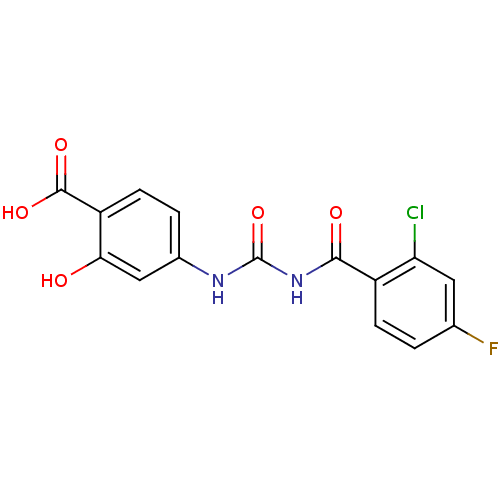 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174398 (4-[3-(2,4-Dichloro-benzoyl)-ureido]-3-methoxy-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate upon incubating for 40 min at 25 de... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50174404 (4-[3-(2-Chloro-4-fluoro-benzoyl)-ureido]-2-hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | 7.2 | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Logarithmic inhibitory concentration against human liver glycogen phosphorylase enzyme by the addition of glucose-1-phosphate and incubated for 40 mi... | J Med Chem 48: 6178-93 (2005) Article DOI: 10.1021/jm049034y BindingDB Entry DOI: 10.7270/Q2FT8KJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |