 Found 574 hits with Last Name = 'musch' and Initial = 'd'
Found 574 hits with Last Name = 'musch' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM38019

(US10093646, Compound 1 | US10301278, Example 00003...)Show InChI InChI=1S/C24H34N4O2/c1-18-8-7-9-19-16-20(23(29)26-22(18)19)17-28(15-14-27-12-5-6-13-27)24(30)25-21-10-3-2-4-11-21/h7-9,16,21H,2-6,10-15,17H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50546219
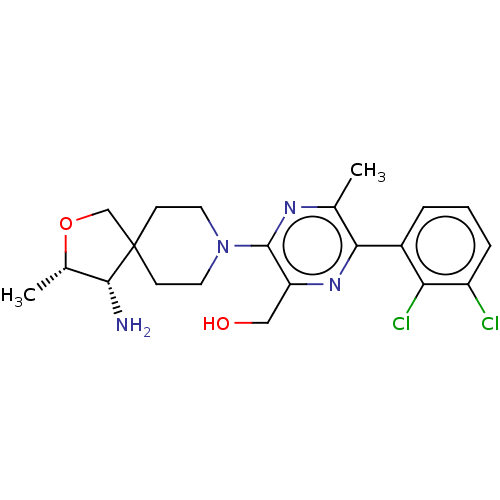
(CHEMBL4752026 | US11596633, Compound B | US1170239...)Show SMILES C[C@@H]1OCC2(CCN(CC2)c2nc(C)c(nc2CO)-c2cccc(Cl)c2Cl)[C@@H]1N |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM608905
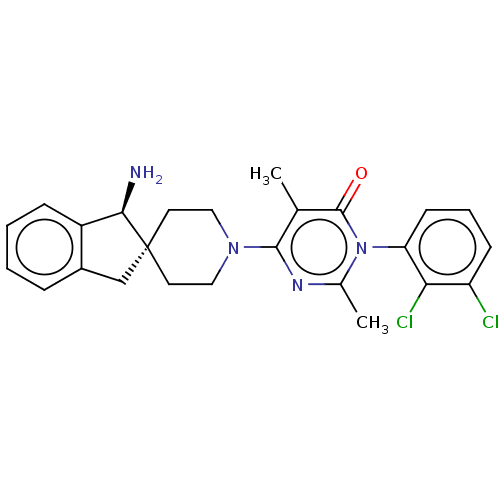
((3M)-6-[(1S)-1-amino-1,3- dihydrospiro[indene-2,4'...)Show SMILES Cc1nc(N2CC[C@]3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wD:7.7,15.17,(-4.02,2.61,;-3.25,1.28,;-1.71,1.28,;-.94,-.05,;.6,-.05,;1.37,1.28,;2.91,1.28,;3.69,-.02,;4.75,-1.14,;6.14,-.48,;7.56,-1.08,;8.79,-.14,;8.59,1.38,;7.17,1.98,;5.94,1.04,;4.43,1.33,;3.77,2.72,;2.91,-1.38,;1.37,-1.38,;-1.71,-1.39,;-.94,-2.72,;-3.25,-1.39,;-4.02,-2.72,;-4.02,-.05,;-5.7,-.06,;-6.47,-1.39,;-8.01,-1.39,;-8.78,-.06,;-8.01,1.28,;-8.79,2.61,;-6.47,1.28,;-5.71,2.61,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM497142

(US11001561, Compound 117 | US11702392, Compound 11...)Show SMILES Cc1c(nc(N)n(-c2cccc(Cl)c2Cl)c1=O)N1CCC2(Cc3ccccc3[C@H]2N)CC1 |r,wD:28.32,(-1.62,-3.11,;-2.01,-1.62,;-.93,-.53,;-1.32,.95,;-2.81,1.35,;-3.21,2.84,;-3.9,.26,;-5.64,.72,;-6.73,-.37,;-8.22,.03,;-8.62,1.51,;-7.53,2.6,;-7.93,4.09,;-6.04,2.2,;-4.95,3.29,;-3.5,-1.22,;-4.59,-2.31,;.56,-.93,;.96,-2.42,;2.45,-2.82,;3.54,-1.73,;4.13,-3.15,;5.66,-3.03,;6.78,-4.09,;8.26,-3.65,;8.62,-2.15,;7.5,-1.09,;6.02,-1.53,;4.71,-.73,;4.71,.81,;3.14,-.24,;1.65,.16,)| Show InChI InChI=1S/C24H25Cl2N5O/c1-14-21(29-23(28)31(22(14)32)18-8-4-7-17(25)19(18)26)30-11-9-24(10-12-30)13-15-5-2-3-6-16(15)20(24)27/h2-8,20H,9-13,27H2,1H3,(H2,28,29)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM497078
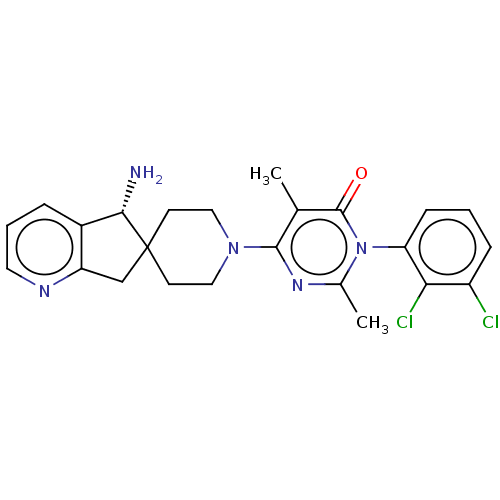
(US11001561, Compound 66b | US11702392, Compound 66...)Show SMILES Cc1nc(N2CCC3(Cc4ncccc4[C@@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wU:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C24H25Cl2N5O/c1-14-22(29-15(2)31(23(14)32)19-7-3-6-17(25)20(19)26)30-11-8-24(9-12-30)13-18-16(21(24)27)5-4-10-28-18/h3-7,10,21H,8-9,11-13,27H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM608823

((3M)-6-[(3S,4S)-4-amino-3- methyl-2-oxa-8- azaspir...)Show SMILES C[C@@H]1OC[C@]2(CCN(CC2)c2cc(=O)n(c(C)n2)-c2cccc(Cl)c2Cl)[C@@H]1N |r,wU:4.3,wD:26.30,1.0,(-7.97,-1.65,;-6.72,-.75,;-6.72,.79,;-5.26,1.27,;-4.35,.02,;-3.58,1.36,;-2.04,1.36,;-1.27,.02,;-2.04,-1.31,;-3.58,-1.31,;.27,.02,;1.04,1.36,;2.58,1.36,;3.35,2.69,;3.35,.02,;2.58,-1.31,;3.35,-2.65,;1.04,-1.31,;4.89,.02,;5.66,-1.31,;7.2,-1.31,;7.97,.02,;7.2,1.36,;7.97,2.69,;5.66,1.36,;4.89,2.69,;-5.26,-1.22,;-4.78,-2.69,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM608845
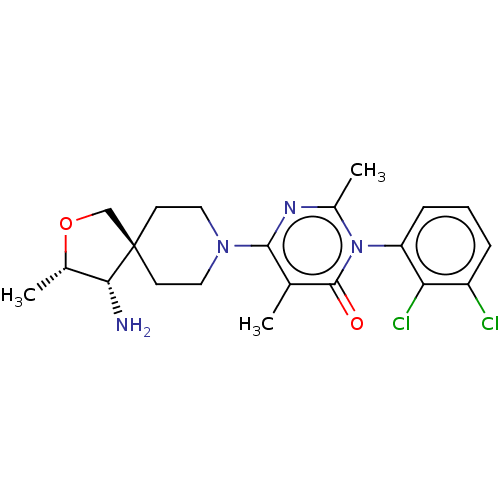
((3M)-6-[(3S,4S)-4-amino-3- methyl-2-oxa-8- azaspir...)Show SMILES C[C@@H]1OC[C@]2(CCN(CC2)c2nc(C)n(-c3cccc(Cl)c3Cl)c(=O)c2C)[C@@H]1N |r,wU:4.3,wD:27.31,1.0,(-7.97,-1.65,;-6.72,-.75,;-6.72,.79,;-5.26,1.27,;-4.35,.02,;-3.58,1.36,;-2.04,1.36,;-1.27,.02,;-2.04,-1.31,;-3.58,-1.31,;.27,.02,;1.04,-1.31,;2.58,-1.31,;3.35,-2.65,;3.35,.02,;4.89,.02,;5.66,-1.31,;7.2,-1.31,;7.97,.02,;7.2,1.36,;7.97,2.69,;5.66,1.36,;4.89,2.69,;2.58,1.36,;3.35,2.69,;1.04,1.36,;.27,2.69,;-5.26,-1.22,;-4.78,-2.69,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497105
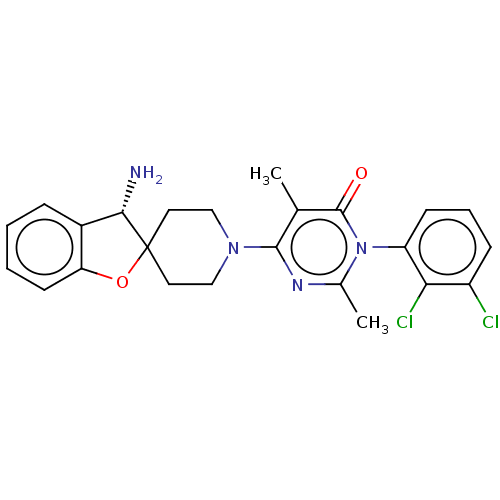
(US11001561, Compound 81a | US11702392, Compound 81...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ccccc2[C@@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C24H24Cl2N4O2/c1-14-22(28-15(2)30(23(14)31)18-8-5-7-17(25)20(18)26)29-12-10-24(11-13-29)21(27)16-6-3-4-9-19(16)32-24/h3-9,21H,10-13,27H2,1-2H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [E76Z]
(Homo sapiens) | BDBM497105

(US11001561, Compound 81a | US11702392, Compound 81...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ccccc2[C@@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C24H24Cl2N4O2/c1-14-22(28-15(2)30(23(14)31)18-8-5-7-17(25)20(18)26)29-12-10-24(11-13-29)21(27)16-6-3-4-9-19(16)32-24/h3-9,21H,10-13,27H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497078

(US11001561, Compound 66b | US11702392, Compound 66...)Show SMILES Cc1nc(N2CCC3(Cc4ncccc4[C@@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wU:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C24H25Cl2N5O/c1-14-22(29-15(2)31(23(14)32)19-7-3-6-17(25)20(19)26)30-11-8-24(9-12-30)13-18-16(21(24)27)5-4-10-28-18/h3-7,10,21H,8-9,11-13,27H2,1-2H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497145

(US11001561, Compound 119b | US11702392, Compound 1...)Show SMILES Cc1nc(cc(=O)n1-c1cccc(Cl)c1Cl)N1CCC2(Cc3ccccc3[C@@H]2N)CC1 |wU:27.31,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;.11,-5.1,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,)| Show InChI InChI=1S/C24H24Cl2N4O/c1-15-28-20(13-21(31)30(15)19-8-4-7-18(25)22(19)26)29-11-9-24(10-12-29)14-16-5-2-3-6-17(16)23(24)27/h2-8,13,23H,9-12,14,27H2,1H3/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497145

(US11001561, Compound 119b | US11702392, Compound 1...)Show SMILES Cc1nc(cc(=O)n1-c1cccc(Cl)c1Cl)N1CCC2(Cc3ccccc3[C@@H]2N)CC1 |wU:27.31,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;.11,-5.1,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,)| Show InChI InChI=1S/C24H24Cl2N4O/c1-15-28-20(13-21(31)30(15)19-8-4-7-18(25)22(19)26)29-11-9-24(10-12-29)14-16-5-2-3-6-17(16)23(24)27/h2-8,13,23H,9-12,14,27H2,1H3/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM503414

((5P)-6-amino-2-[(1S)-1-amino-1,3- dihydrospiro[ind...)Show SMILES N[C@@H]1c2ccccc2CC11CCN(CC1)c1nc(N)c(C2=C(Cl)C(Cl)=CCC2)c(n1)C(N)=O |r,c:23,27| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930X9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM503416

((5M)-2-[(1S)-1-amino-1,3- dihydrospiro[indene-2,4'...)Show SMILES N[C@H]1c2cccnc2OC11CCN(CC1)c1nc(N)c(-c2cccc(Cl)c2Cl)c(n1)C(N)=O |r,wU:1.0,(4.07,3.3,;4.55,1.84,;6.01,1.36,;7.34,2.13,;8.68,1.36,;8.68,-.18,;7.34,-.95,;6.01,-.18,;4.55,-.66,;3.64,.59,;2.87,1.92,;1.33,1.92,;.56,.59,;1.33,-.74,;2.87,-.74,;-.98,.59,;-1.75,1.92,;-3.29,1.92,;-4.06,3.26,;-4.06,.59,;-5.6,.59,;-6.37,-.74,;-7.91,-.74,;-8.68,.59,;-7.91,1.92,;-8.68,3.26,;-6.37,1.92,;-6.77,3.41,;-3.29,-.74,;-1.75,-.74,;-4.06,-2.08,;-3.29,-3.41,;-5.6,-2.08,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930X9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM608512
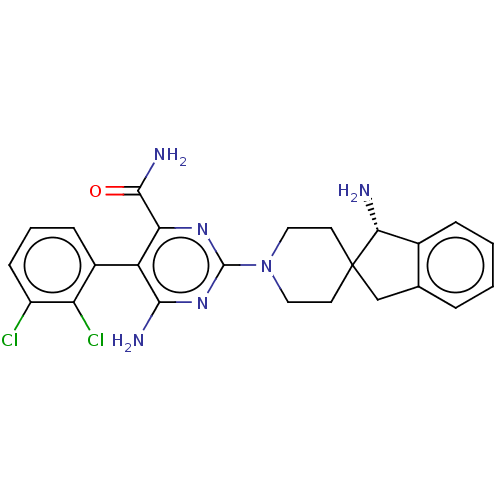
((5P)-6-amino-2-[(1S)-1-amino-1,3- dihydrospiro[ind...)Show SMILES N[C@@H]1c2ccccc2CC11CCN(CC1)c1nc(N)c(-c2cccc(Cl)c2Cl)c(n1)C(N)=O |r,wD:1.0,(5.14,-.68,;4.37,-2.02,;5.4,-3.16,;6.94,-3.16,;7.71,-4.49,;6.94,-5.83,;5.4,-5.83,;4.63,-4.49,;3.12,-4.17,;2.96,-2.64,;1.63,-3.41,;.29,-2.64,;.29,-1.1,;1.63,-.33,;2.96,-1.1,;-1.04,-.33,;-1.04,1.21,;-2.37,1.98,;-2.37,3.52,;-3.71,1.21,;-5.04,1.98,;-6.37,1.21,;-7.71,1.98,;-7.71,3.52,;-6.37,4.29,;-6.37,5.83,;-5.04,3.52,;-3.71,4.29,;-3.71,-.33,;-2.37,-1.1,;-5.04,-1.1,;-5.04,-2.64,;-6.37,-.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JS9VKW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497078

(US11001561, Compound 66b | US11702392, Compound 66...)Show SMILES Cc1nc(N2CCC3(Cc4ncccc4[C@@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wU:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C24H25Cl2N5O/c1-14-22(29-15(2)31(23(14)32)19-7-3-6-17(25)20(19)26)30-11-8-24(9-12-30)13-18-16(21(24)27)5-4-10-28-18/h3-7,10,21H,8-9,11-13,27H2,1-2H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497088
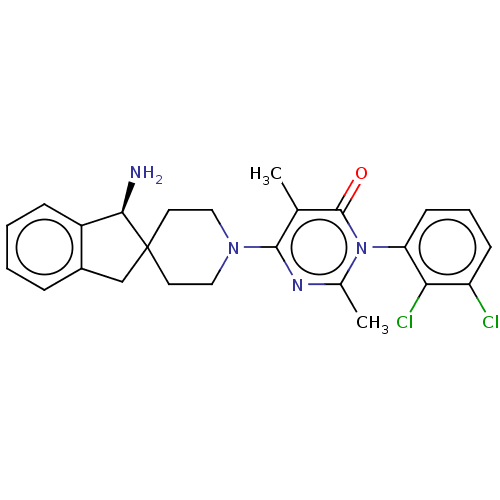
(US11001561, Compound 74a | US11702392, Compound 10...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wD:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C25H26Cl2N4O/c1-15-23(29-16(2)31(24(15)32)20-9-5-8-19(26)21(20)27)30-12-10-25(11-13-30)14-17-6-3-4-7-18(17)22(25)28/h3-9,22H,10-14,28H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM608905

((3M)-6-[(1S)-1-amino-1,3- dihydrospiro[indene-2,4'...)Show SMILES Cc1nc(N2CC[C@]3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wD:7.7,15.17,(-4.02,2.61,;-3.25,1.28,;-1.71,1.28,;-.94,-.05,;.6,-.05,;1.37,1.28,;2.91,1.28,;3.69,-.02,;4.75,-1.14,;6.14,-.48,;7.56,-1.08,;8.79,-.14,;8.59,1.38,;7.17,1.98,;5.94,1.04,;4.43,1.33,;3.77,2.72,;2.91,-1.38,;1.37,-1.38,;-1.71,-1.39,;-.94,-2.72,;-3.25,-1.39,;-4.02,-2.72,;-4.02,-.05,;-5.7,-.06,;-6.47,-1.39,;-8.01,-1.39,;-8.78,-.06,;-8.01,1.28,;-8.79,2.61,;-6.47,1.28,;-5.71,2.61,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM608514
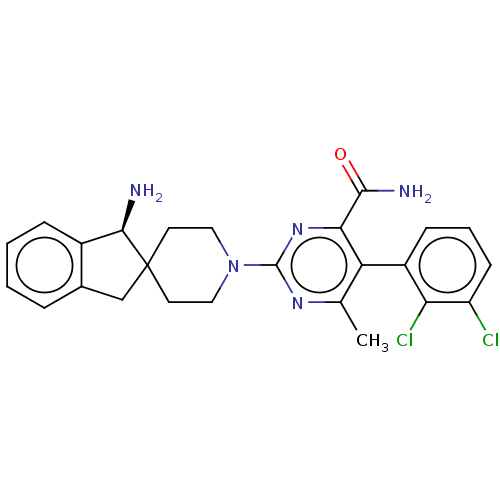
((5M)-2-[(1S)-1-amino-1,3- dihydrospiro[indene-2,4'...)Show SMILES Cc1nc(nc(C(N)=O)c1-c1cccc(Cl)c1Cl)N1CCC2(Cc3ccccc3[C@H]2N)CC1 |r,wD:29.33,(-2.37,3.52,;-2.37,1.98,;-1.04,1.21,;-1.04,-.33,;-2.37,-1.1,;-3.71,-.33,;-5.04,-1.1,;-5.04,-2.64,;-6.37,-.33,;-3.71,1.21,;-5.04,1.98,;-6.37,1.21,;-7.71,1.98,;-7.71,3.52,;-6.37,4.29,;-6.37,5.83,;-5.04,3.52,;-3.71,4.29,;.29,-1.1,;.29,-2.64,;1.63,-3.41,;2.96,-2.64,;3.12,-4.17,;4.63,-4.49,;5.4,-5.83,;6.94,-5.83,;7.71,-4.49,;6.94,-3.16,;5.4,-3.16,;4.37,-2.02,;5.14,-.68,;2.96,-1.1,;1.63,-.33,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JS9VKW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497078

(US11001561, Compound 66b | US11702392, Compound 66...)Show SMILES Cc1nc(N2CCC3(Cc4ncccc4[C@@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wU:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C24H25Cl2N5O/c1-14-22(29-15(2)31(23(14)32)19-7-3-6-17(25)20(19)26)30-11-8-24(9-12-30)13-18-16(21(24)27)5-4-10-28-18/h3-7,10,21H,8-9,11-13,27H2,1-2H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [E76Z]
(Homo sapiens) | BDBM497105

(US11001561, Compound 81a | US11702392, Compound 81...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ccccc2[C@@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C24H24Cl2N4O2/c1-14-22(28-15(2)30(23(14)31)18-8-5-7-17(25)20(18)26)29-12-10-24(11-13-29)21(27)16-6-3-4-9-19(16)32-24/h3-9,21H,10-13,27H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497105

(US11001561, Compound 81a | US11702392, Compound 81...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ccccc2[C@@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C24H24Cl2N4O2/c1-14-22(28-15(2)30(23(14)31)18-8-5-7-17(25)20(18)26)29-12-10-24(11-13-29)21(27)16-6-3-4-9-19(16)32-24/h3-9,21H,10-13,27H2,1-2H3/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497106
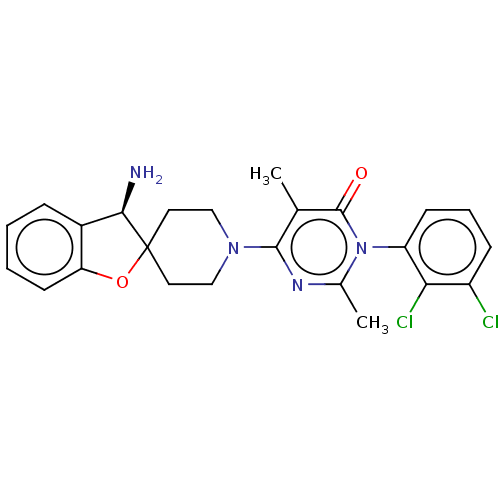
(US11001561, Compound 81b | US11702392, Compound 81...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ccccc2[C@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wU:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C24H24Cl2N4O2/c1-14-22(28-15(2)30(23(14)31)18-8-5-7-17(25)20(18)26)29-12-10-24(11-13-29)21(27)16-6-3-4-9-19(16)32-24/h3-9,21H,10-13,27H2,1-2H3/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497088

(US11001561, Compound 74a | US11702392, Compound 10...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wD:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C25H26Cl2N4O/c1-15-23(29-16(2)31(24(15)32)20-9-5-8-19(26)21(20)27)30-12-10-25(11-13-30)14-17-6-3-4-7-18(17)22(25)28/h3-9,22H,10-14,28H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [E76Z]
(Homo sapiens) | BDBM497078

(US11001561, Compound 66b | US11702392, Compound 66...)Show SMILES Cc1nc(N2CCC3(Cc4ncccc4[C@@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wU:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C24H25Cl2N5O/c1-14-22(29-15(2)31(23(14)32)19-7-3-6-17(25)20(19)26)30-11-8-24(9-12-30)13-18-16(21(24)27)5-4-10-28-18/h3-7,10,21H,8-9,11-13,27H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [E76Z]
(Homo sapiens) | BDBM497078

(US11001561, Compound 66b | US11702392, Compound 66...)Show SMILES Cc1nc(N2CCC3(Cc4ncccc4[C@@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wU:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C24H25Cl2N5O/c1-14-22(29-15(2)31(23(14)32)19-7-3-6-17(25)20(19)26)30-11-8-24(9-12-30)13-18-16(21(24)27)5-4-10-28-18/h3-7,10,21H,8-9,11-13,27H2,1-2H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM503416

((5M)-2-[(1S)-1-amino-1,3- dihydrospiro[indene-2,4'...)Show SMILES N[C@H]1c2cccnc2OC11CCN(CC1)c1nc(N)c(-c2cccc(Cl)c2Cl)c(n1)C(N)=O |r,wU:1.0,(4.07,3.3,;4.55,1.84,;6.01,1.36,;7.34,2.13,;8.68,1.36,;8.68,-.18,;7.34,-.95,;6.01,-.18,;4.55,-.66,;3.64,.59,;2.87,1.92,;1.33,1.92,;.56,.59,;1.33,-.74,;2.87,-.74,;-.98,.59,;-1.75,1.92,;-3.29,1.92,;-4.06,3.26,;-4.06,.59,;-5.6,.59,;-6.37,-.74,;-7.91,-.74,;-8.68,.59,;-7.91,1.92,;-8.68,3.26,;-6.37,1.92,;-6.77,3.41,;-3.29,-.74,;-1.75,-.74,;-4.06,-2.08,;-3.29,-3.41,;-5.6,-2.08,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930X9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497134

(US11001561, Compound 109 | US11702392, Compound 10...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1ccc(F)c(Cl)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-8.36,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C25H25Cl2FN4O/c1-14-23(30-15(2)32(24(14)33)19-8-7-18(28)20(26)21(19)27)31-11-9-25(10-12-31)13-16-5-3-4-6-17(16)22(25)29/h3-8,22H,9-13,29H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM608516
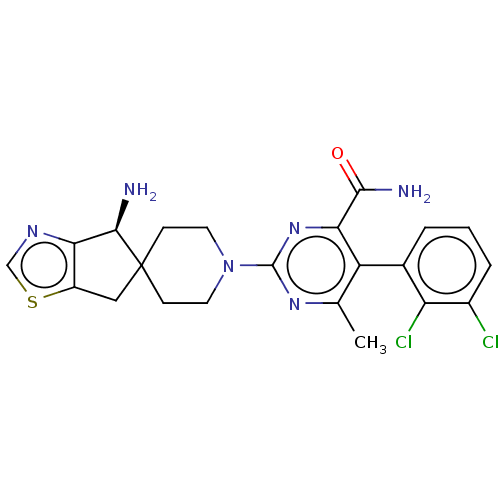
(6-amino-2-[(4S)-4-amino-4,6- dihydrospiro[cyclopen...)Show SMILES Cc1nc(nc(C(N)=O)c1-c1cccc(Cl)c1Cl)N1CCC2(Cc3scnc3[C@H]2N)CC1 |r,wD:28.32,(-1.92,3.56,;-1.92,2.02,;-.58,1.25,;-.58,-.29,;-1.92,-1.06,;-3.25,-.29,;-4.58,-1.06,;-4.58,-2.6,;-5.92,-.29,;-3.25,1.25,;-4.58,2.02,;-5.92,1.25,;-7.25,2.02,;-7.25,3.56,;-5.92,4.33,;-5.92,5.87,;-4.58,3.56,;-3.25,4.33,;.75,-1.06,;.75,-2.6,;2.08,-3.37,;3.42,-2.6,;3.42,-4.14,;4.88,-4.62,;5.79,-5.87,;7.25,-5.39,;7.25,-3.85,;5.79,-3.37,;4.88,-2.13,;5.65,-.79,;3.42,-1.06,;2.08,-.29,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JS9VKW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497134

(US11001561, Compound 109 | US11702392, Compound 10...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1ccc(F)c(Cl)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-8.36,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C25H25Cl2FN4O/c1-14-23(30-15(2)32(24(14)33)19-8-7-18(28)20(26)21(19)27)31-11-9-25(10-12-31)13-16-5-3-4-6-17(16)22(25)29/h3-8,22H,9-13,29H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497135

(US11001561, Compound 110 | US11702392, Compound 11...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1ccc(F)c(C)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-8.36,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C26H28ClFN4O/c1-15-20(28)8-9-21(22(15)27)32-17(3)30-24(16(2)25(32)33)31-12-10-26(11-13-31)14-18-6-4-5-7-19(18)23(26)29/h4-9,23H,10-14,29H2,1-3H3/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497135

(US11001561, Compound 110 | US11702392, Compound 11...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1ccc(F)c(C)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-8.36,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C26H28ClFN4O/c1-15-20(28)8-9-21(22(15)27)32-17(3)30-24(16(2)25(32)33)31-12-10-26(11-13-31)14-18-6-4-5-7-19(18)23(26)29/h4-9,23H,10-14,29H2,1-3H3/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [E76Z]
(Homo sapiens) | BDBM497088

(US11001561, Compound 74a | US11702392, Compound 10...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wD:15.17,(-3.34,2.87,;-2.94,1.38,;-1.45,.98,;-1.05,-.51,;.44,-.91,;.83,-2.39,;2.32,-2.79,;3.41,-1.7,;4,-3.13,;5.54,-3,;6.65,-4.06,;8.13,-3.62,;8.49,-2.13,;7.37,-1.07,;5.89,-1.51,;4.58,-.7,;4.58,.84,;3.01,-.22,;1.52,.18,;-2.14,-1.6,;-1.74,-3.08,;-3.63,-1.2,;-4.72,-2.29,;-4.03,.29,;-5.51,.69,;-6.6,-.4,;-8.09,-0,;-8.49,1.49,;-7.4,2.57,;-7.8,4.06,;-5.91,2.18,;-4.82,3.26,)| Show InChI InChI=1S/C25H26Cl2N4O/c1-15-23(29-16(2)31(24(15)32)20-9-5-8-19(26)21(20)27)30-12-10-25(11-13-30)14-17-6-3-4-7-18(17)22(25)28/h3-9,22H,10-14,28H2,1-2H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497137
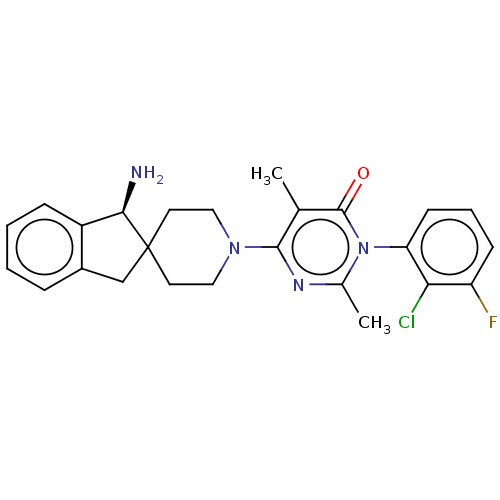
(US11001561, Compound 112 | US11702392, Compound 11...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(F)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C25H26ClFN4O/c1-15-23(29-16(2)31(24(15)32)20-9-5-8-19(27)21(20)26)30-12-10-25(11-13-30)14-17-6-3-4-7-18(17)22(25)28/h3-9,22H,10-14,28H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497137

(US11001561, Compound 112 | US11702392, Compound 11...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(F)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C25H26ClFN4O/c1-15-23(29-16(2)31(24(15)32)20-9-5-8-19(27)21(20)26)30-12-10-25(11-13-30)14-17-6-3-4-7-18(17)22(25)28/h3-9,22H,10-14,28H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497101

(US11001561, Compound 80a | US11001561, Compound 80...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2cnccc2[C@@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C23H23Cl2N5O2/c1-13-21(28-14(2)30(22(13)31)17-5-3-4-16(24)19(17)25)29-10-7-23(8-11-29)20(26)15-6-9-27-12-18(15)32-23/h3-6,9,12,20H,7-8,10-11,26H2,1-2H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497129

(US11001561, Compound 104 | US11702392, Compound 10...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(Br)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C25H26BrClN4O/c1-15-23(29-16(2)31(24(15)32)20-9-5-8-19(26)21(20)27)30-12-10-25(11-13-30)14-17-6-3-4-7-18(17)22(25)28/h3-9,22H,10-14,28H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM608920

((3M)-6-[(3R)-3-amino-3H- spiro[furo[2,3-c]pyridine...)Show SMILES Cc1nc(N2CC[C@@]3(CC2)Oc2cnccc2[C@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |r,wD:7.10,17.20,(-4.01,2.64,;-3.23,1.31,;-1.69,1.31,;-.92,-.02,;.62,-.02,;1.39,1.31,;2.93,1.32,;3.7,-.02,;2.93,-1.35,;1.39,-1.35,;4.6,-1.26,;6.07,-.78,;7.4,-1.55,;8.73,-.78,;8.73,.76,;7.4,1.53,;6.07,.76,;4.6,1.23,;4.12,2.69,;-1.69,-1.36,;-.92,-2.69,;-3.23,-1.36,;-4,-2.69,;-4,-.03,;-5.65,-.03,;-6.42,-1.36,;-7.96,-1.37,;-8.73,-.03,;-7.96,1.3,;-8.73,2.63,;-6.42,1.3,;-5.65,2.64,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497129

(US11001561, Compound 104 | US11702392, Compound 10...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(Br)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C25H26BrClN4O/c1-15-23(29-16(2)31(24(15)32)20-9-5-8-19(26)21(20)27)30-12-10-25(11-13-30)14-17-6-3-4-7-18(17)22(25)28/h3-9,22H,10-14,28H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM503416

((5M)-2-[(1S)-1-amino-1,3- dihydrospiro[indene-2,4'...)Show SMILES N[C@H]1c2cccnc2OC11CCN(CC1)c1nc(N)c(-c2cccc(Cl)c2Cl)c(n1)C(N)=O |r,wU:1.0,(4.07,3.3,;4.55,1.84,;6.01,1.36,;7.34,2.13,;8.68,1.36,;8.68,-.18,;7.34,-.95,;6.01,-.18,;4.55,-.66,;3.64,.59,;2.87,1.92,;1.33,1.92,;.56,.59,;1.33,-.74,;2.87,-.74,;-.98,.59,;-1.75,1.92,;-3.29,1.92,;-4.06,3.26,;-4.06,.59,;-5.6,.59,;-6.37,-.74,;-7.91,-.74,;-8.68,.59,;-7.91,1.92,;-8.68,3.26,;-6.37,1.92,;-6.77,3.41,;-3.29,-.74,;-1.75,-.74,;-4.06,-2.08,;-3.29,-3.41,;-5.6,-2.08,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930X9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM608523

((4P)-6-amino-2-[(3R)-3-amino-3H- spiro[furo[2,3-b]...)Show SMILES N[C@@H]1c2cccnc2OC11CCN(CC1)c1nc(N)c(-c2cccc(Cl)c2Cl)c(n1)C(N)=O |r,wD:1.0,(4,3.22,;4.77,1.89,;6.23,1.41,;7.57,2.18,;8.9,1.41,;8.9,-.13,;7.57,-.9,;6.23,-.13,;4.77,-.6,;3.86,.64,;3.09,-.69,;1.55,-.69,;.78,.64,;1.55,1.98,;3.09,1.98,;-.76,.64,;-1.53,1.98,;-3.07,1.98,;-3.84,3.31,;-3.84,.64,;-5.82,.64,;-6.59,-.69,;-8.13,-.69,;-8.9,.64,;-8.13,1.98,;-8.9,3.31,;-6.59,1.98,;-7.14,3.36,;-3.07,-.69,;-1.53,-.69,;-3.84,-2.02,;-3.07,-3.36,;-5.38,-2.02,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JS9VKW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497100

(US11001561, Compound 79b | US11702392, Compound 79...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ncccc2[C@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wU:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C23H23Cl2N5O2/c1-13-20(28-14(2)30(22(13)31)17-7-3-6-16(24)18(17)25)29-11-8-23(9-12-29)19(26)15-5-4-10-27-21(15)32-23/h3-7,10,19H,8-9,11-12,26H2,1-2H3/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497130

(US11001561, Compound 105 | US11702392, Compound 10...)Show SMILES COc1c(F)ccc(c1F)-n1c(C)nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c1=O |wD:26.29,(-8.36,-6.44,;-6.82,-6.44,;-6.05,-5.1,;-6.82,-3.77,;-8.36,-3.77,;-6.05,-2.44,;-4.51,-2.44,;-3.74,-3.77,;-4.51,-5.1,;-3.74,-6.44,;-2.2,-3.77,;-1.43,-2.44,;-2.2,-1.1,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,)| Show InChI InChI=1S/C26H28F2N4O2/c1-15-24(30-16(2)32(25(15)33)20-9-8-19(27)22(34-3)21(20)28)31-12-10-26(11-13-31)14-17-6-4-5-7-18(17)23(26)29/h4-9,23H,10-14,29H2,1-3H3/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497131
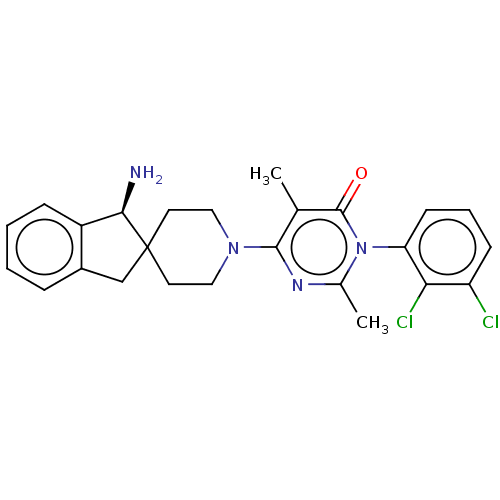
(US11001561, Compound 106)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C25H26Cl2N4O/c1-15-23(29-16(2)31(24(15)32)20-9-5-8-19(26)21(20)27)30-12-10-25(11-13-30)14-17-6-3-4-7-18(17)22(25)28/h3-9,22H,10-14,28H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497138

(US11001561, Compound 113 | US11702392, Compound 11...)Show SMILES Cc1nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c(=O)n1-c1ccc(F)c(F)c1Cl |wD:15.17,(-2.2,-1.1,;-1.43,-2.44,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,;-2.2,-3.77,;-3.74,-3.77,;-4.51,-2.44,;-6.05,-2.44,;-6.82,-3.77,;-8.36,-3.77,;-6.05,-5.1,;-6.82,-6.44,;-4.51,-5.1,;-3.74,-6.44,)| Show InChI InChI=1S/C25H25ClF2N4O/c1-14-23(30-15(2)32(24(14)33)19-8-7-18(27)21(28)20(19)26)31-11-9-25(10-12-31)13-16-5-3-4-6-17(16)22(25)29/h3-8,22H,9-13,29H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497094

(US11001561, Compound 77)Show SMILES C[C@H]1C[C@@H](N)C2(C1)CCN(CC2)c1nc(C)n(C2CCCC(Cl)=C2Cl)c(=O)c1C |r,c:24| Show InChI InChI=1S/C22H32Cl2N4O/c1-13-11-18(25)22(12-13)7-9-27(10-8-22)20-14(2)21(29)28(15(3)26-20)17-6-4-5-16(23)19(17)24/h13,17-18H,4-12,25H2,1-3H3/t13-,17?,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Patent GmbH
US Patent
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
US Patent US11001561 (2021)
BindingDB Entry DOI: 10.7270/Q2BC42PT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM503416

((5M)-2-[(1S)-1-amino-1,3- dihydrospiro[indene-2,4'...)Show SMILES N[C@H]1c2cccnc2OC11CCN(CC1)c1nc(N)c(-c2cccc(Cl)c2Cl)c(n1)C(N)=O |r,wU:1.0,(4.07,3.3,;4.55,1.84,;6.01,1.36,;7.34,2.13,;8.68,1.36,;8.68,-.18,;7.34,-.95,;6.01,-.18,;4.55,-.66,;3.64,.59,;2.87,1.92,;1.33,1.92,;.56,.59,;1.33,-.74,;2.87,-.74,;-.98,.59,;-1.75,1.92,;-3.29,1.92,;-4.06,3.26,;-4.06,.59,;-5.6,.59,;-6.37,-.74,;-7.91,-.74,;-8.68,.59,;-7.91,1.92,;-8.68,3.26,;-6.37,1.92,;-6.77,3.41,;-3.29,-.74,;-1.75,-.74,;-4.06,-2.08,;-3.29,-3.41,;-5.6,-2.08,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930X9N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM497130

(US11001561, Compound 105 | US11702392, Compound 10...)Show SMILES COc1c(F)ccc(c1F)-n1c(C)nc(N2CCC3(Cc4ccccc4[C@H]3N)CC2)c(C)c1=O |wD:26.29,(-8.36,-6.44,;-6.82,-6.44,;-6.05,-5.1,;-6.82,-3.77,;-8.36,-3.77,;-6.05,-2.44,;-4.51,-2.44,;-3.74,-3.77,;-4.51,-5.1,;-3.74,-6.44,;-2.2,-3.77,;-1.43,-2.44,;-2.2,-1.1,;.11,-2.44,;.88,-3.77,;2.42,-3.77,;3.19,-5.1,;4.73,-5.1,;5.5,-3.77,;6.4,-5.02,;7.87,-4.54,;9.2,-5.31,;10.53,-4.54,;10.53,-3,;9.2,-2.23,;7.87,-3,;6.4,-2.53,;5.92,-1.06,;4.73,-2.44,;3.19,-2.44,;.11,-5.1,;.88,-6.44,;-1.43,-5.1,;-2.2,-6.44,)| Show InChI InChI=1S/C26H28F2N4O2/c1-15-24(30-16(2)32(25(15)33)20-9-8-19(27)22(34-3)21(20)28)31-12-10-26(11-13-31)14-17-6-4-5-7-18(17)23(26)29/h4-9,23H,10-14,29H2,1-3H3/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM608911

((3M)-6-[(1R,3R)-1-amino-3- methyl-8-azaspiro[4.5]d...)Show SMILES C[C@H]1C[C@@H](N)C2(C1)CCN(CC2)c1nc(C)n(-c2cccc(Cl)c2Cl)c(=O)c1C |r,wD:3.3,1.0,(8.02,-1.69,;6.78,-.79,;6.78,.75,;5.31,1.23,;4.84,2.69,;4.41,-.02,;5.31,-1.26,;3.64,-1.35,;2.1,-1.35,;1.33,-.02,;2.1,1.31,;3.64,1.31,;-.21,-.02,;-.98,1.31,;-2.52,1.31,;-3.3,2.64,;-3.29,-.02,;-4.94,-.03,;-5.71,-1.36,;-7.25,-1.36,;-8.02,-.03,;-7.25,1.31,;-8.02,2.64,;-5.71,1.31,;-4.94,2.64,;-2.52,-1.36,;-3.29,-2.69,;-.98,-1.36,;-.21,-2.69,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
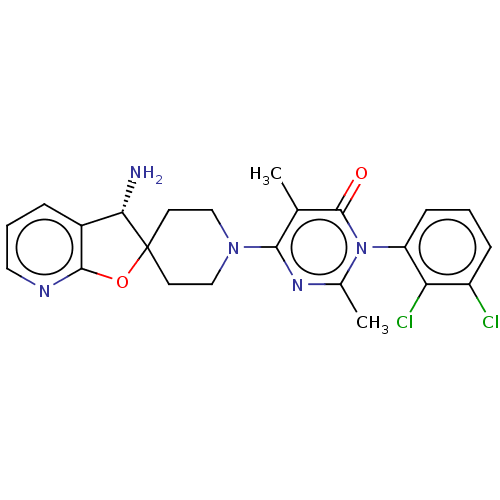
(Homo sapiens (Human)) | BDBM497099
(US11001561, Compound 79a | US11702392, Compound 79...)Show SMILES Cc1nc(N2CCC3(CC2)Oc2ncccc2[C@@H]3N)c(C)c(=O)n1-c1cccc(Cl)c1Cl |wD:17.20,(-7.45,-1.1,;-6.68,-2.44,;-5.14,-2.44,;-4.37,-3.77,;-2.83,-3.77,;-2.06,-5.1,;-.52,-5.1,;.25,-3.77,;-.52,-2.44,;-2.06,-2.44,;1.16,-2.53,;2.62,-3,;3.96,-2.23,;5.29,-3,;5.29,-4.54,;3.96,-5.31,;2.62,-4.54,;1.16,-5.02,;.68,-6.48,;-5.14,-5.1,;-4.37,-6.44,;-6.68,-5.1,;-7.45,-6.44,;-7.45,-3.77,;-8.99,-3.77,;-9.76,-2.44,;-11.3,-2.44,;-12.07,-3.77,;-11.3,-5.1,;-12.07,-6.44,;-9.76,-5.1,;-8.99,-6.44,)| Show InChI InChI=1S/C23H23Cl2N5O2/c1-13-20(28-14(2)30(22(13)31)17-7-3-6-16(24)18(17)25)29-11-8-23(9-12-29)19(26)15-5-4-10-27-21(15)32-23/h3-7,10,19H,8-9,11-12,26H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2891B0Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data