

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477513 (CHEMBL392246) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477923 (CHEMBL437977) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477516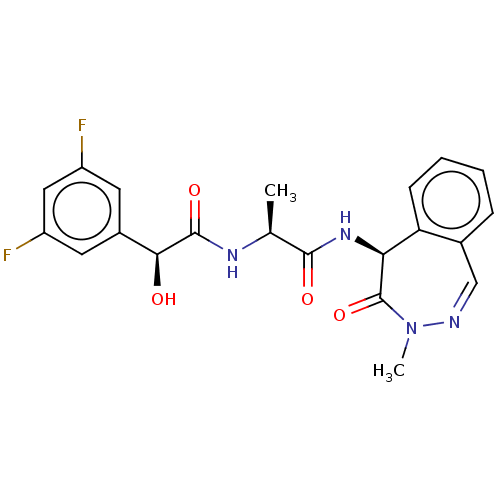 (BMS-433796 | CHEMBL247361) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM29012 (N-[(4R)-4-{[5-chloro-2-(hydroxymethyl)phenyl](4-ch...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50007692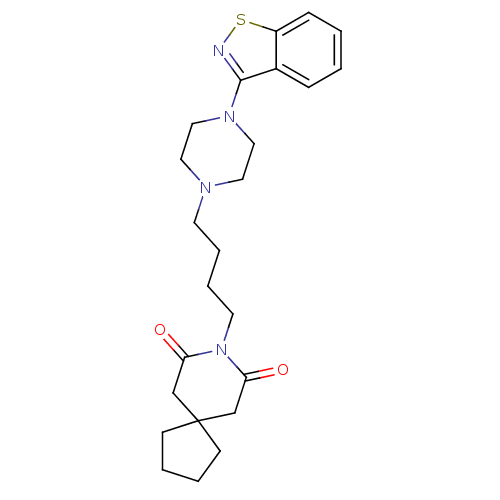 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against rat 5-hydroxytryptamine 2 receptor in rat cerebral cortex tissue using [3H]-spiperone as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50007692 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor measured using radioligand ([3H]spiperone) binding assay | J Med Chem 29: 359-69 (1986) BindingDB Entry DOI: 10.7270/Q24X58C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477528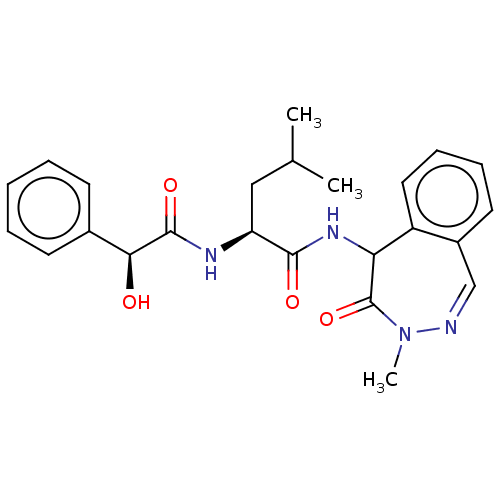 (CHEMBL397844) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477531 (CHEMBL396808) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM29013 (4-chloro-N-[5-chloro-2-(hydroxymethyl)phenyl]-N-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477936 (CHEMBL252052) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477536 (CHEMBL247155) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477922 (CHEMBL252051) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50007693 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.876 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor in rat cerebral cortex tissue using [3H]WB-4101 as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50367315 (CHEMBL1203220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro affinity for cortical alpha-1 adrenergic receptor labelled with [3H]WB-4101 | J Med Chem 29: 359-69 (1986) BindingDB Entry DOI: 10.7270/Q24X58C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477915 (CHEMBL252846) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477526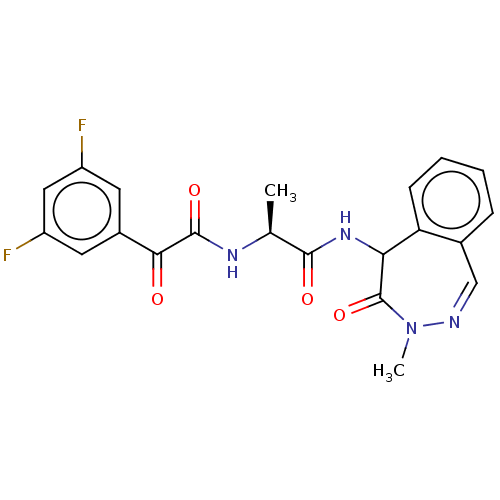 (CHEMBL429292) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50223758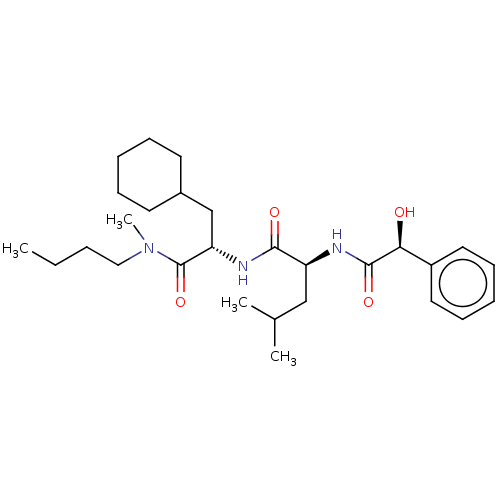 (CHEMBL58925) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase activity in H4 human neuroglioma cells by measuring amyloid beta production | Bioorg Med Chem Lett 14: 1917-21 (2004) BindingDB Entry DOI: 10.7270/Q2TT4T4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50019378 (3-[4-(4-Benzo[d]isoxazol-3-yl-piperazin-1-yl)-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro affinity for cortical alpha-1 adrenergic receptor labelled with [3H]WB-4101 | J Med Chem 29: 359-69 (1986) BindingDB Entry DOI: 10.7270/Q24X58C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477521 (CHEMBL439210) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50125393 (CHEMBL57014) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase activity in H4 human neuroglioma cells by measuring amyloid beta production | Bioorg Med Chem Lett 14: 1917-21 (2004) BindingDB Entry DOI: 10.7270/Q2TT4T4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50007690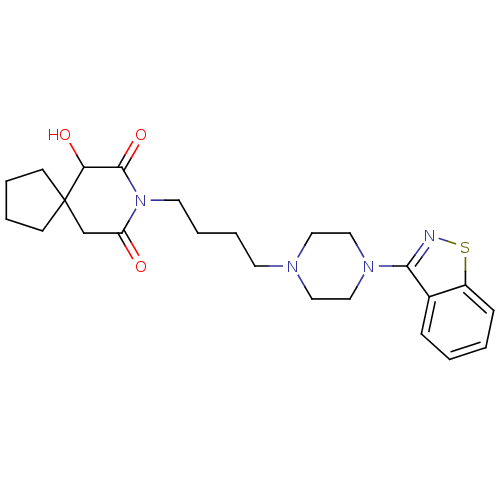 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against rat 5-hydroxytryptamine 2 receptor in rat cerebral cortex tissue using [3H]-spiperone as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477917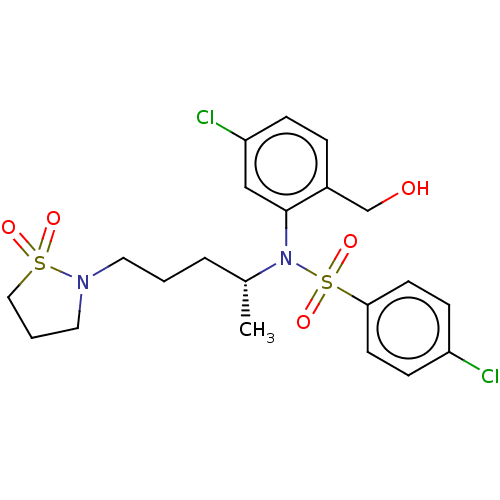 (CHEMBL253295) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477945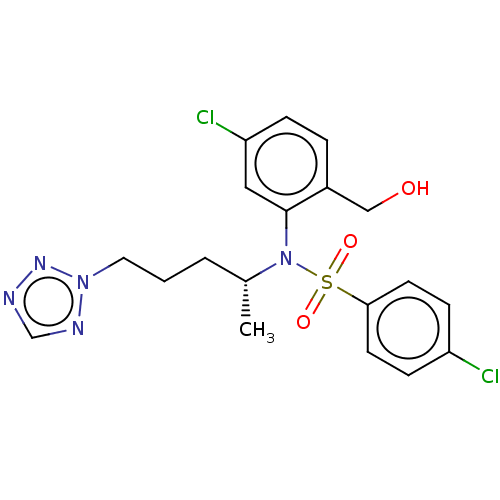 (CHEMBL429838) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477517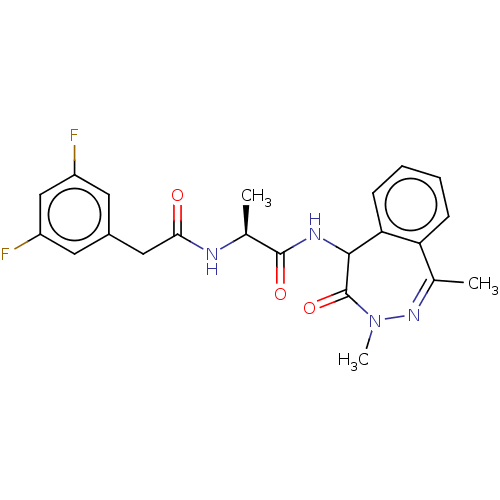 (CHEMBL246785) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50007694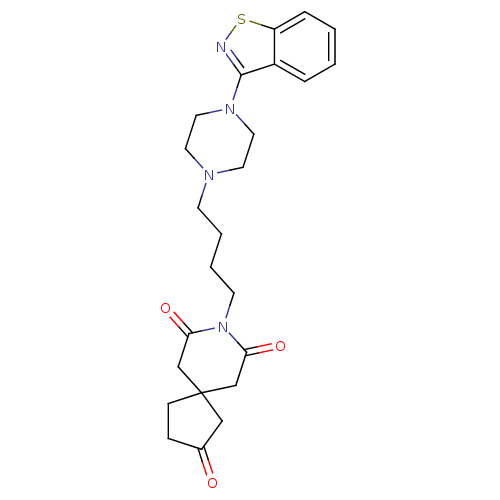 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor in rat cerebral cortex tissue using [3H]WB-4101 as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477932 (CHEMBL253091) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50007690 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor in rat cerebral cortex tissue using [3H]WB-4101 as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477534 (CHEMBL397394) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50019359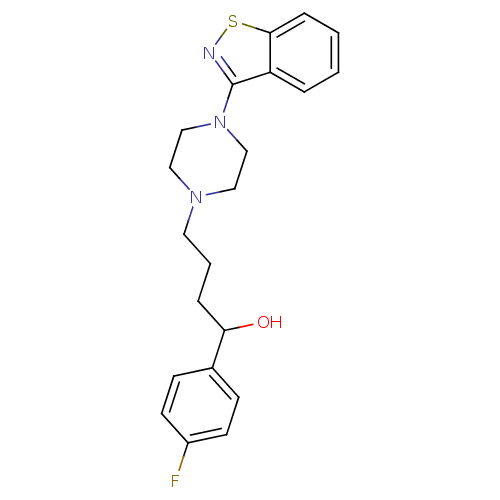 (4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-1-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro affinity for cortical alpha-1 adrenergic receptor labelled with [3H]WB-4101 | J Med Chem 29: 359-69 (1986) BindingDB Entry DOI: 10.7270/Q24X58C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50019376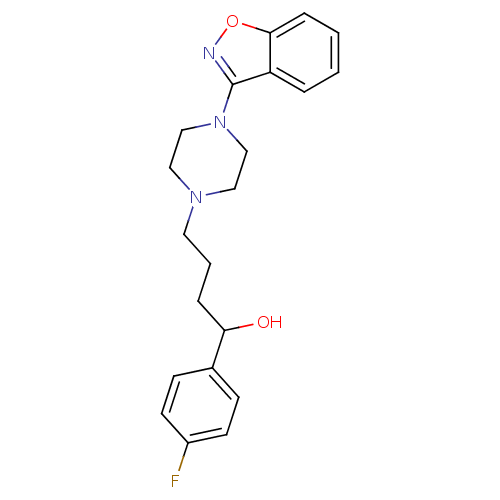 (4-(4-Benzo[d]isoxazol-3-yl-piperazin-1-yl)-1-(4-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro affinity for cortical alpha-1 adrenergic receptor labelled with [3H]WB-4101 | J Med Chem 29: 359-69 (1986) BindingDB Entry DOI: 10.7270/Q24X58C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50019359 (4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-1-(4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro affinity for rat striatal Dopamine receptor D2 labeled with [3H]spiperone | J Med Chem 29: 359-69 (1986) BindingDB Entry DOI: 10.7270/Q24X58C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477519 (CHEMBL397335) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477511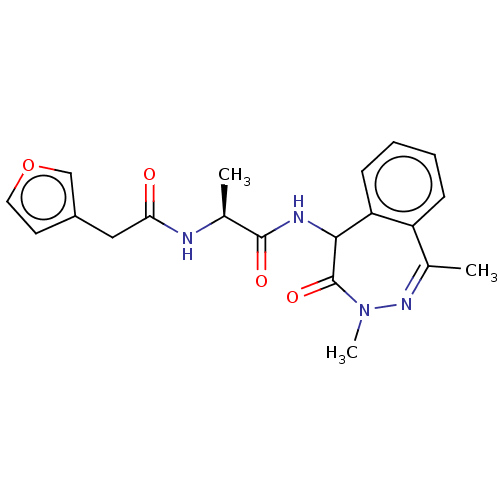 (CHEMBL245385) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50007693 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against rat 5-hydroxytryptamine 2 receptor in rat cerebral cortex tissue using [3H]-spiperone as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50007691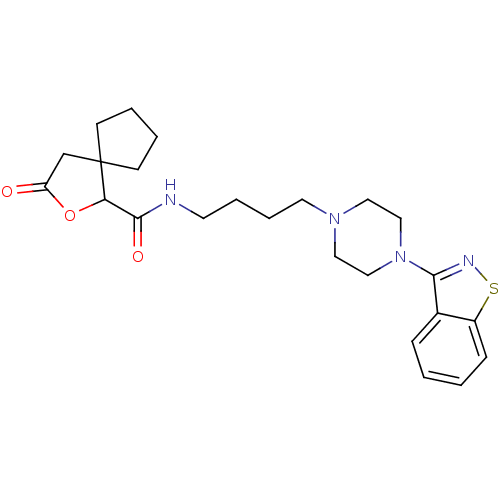 (3-Oxo-2-oxa-spiro[4.4]nonane-1-carboxylic acid [4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor in rat cerebral cortex tissue using [3H]WB-4101 as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50007694 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against rat 5-hydroxytryptamine 2 receptor in rat cerebral cortex tissue using [3H]-spiperone as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007692 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor of rat hippocampal tissue with [3H]-8-OH-DPAT | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477939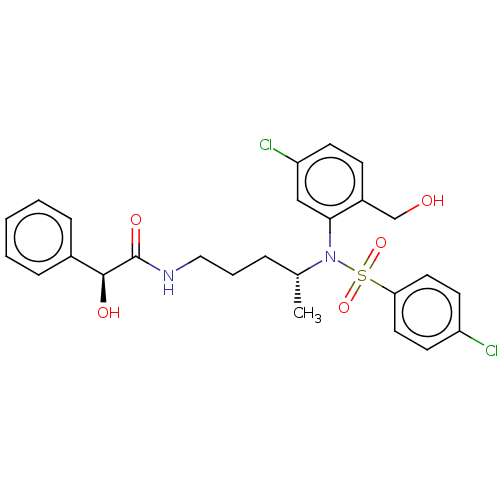 (CHEMBL253092) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007694 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor using in rat hippocampal tissue using [3H]-8-OH-DPAT as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477530 (CHEMBL246575) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50019372 (8-[3-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro affinity for cortical alpha-1 adrenergic receptor labelled with [3H]WB-4101 | J Med Chem 29: 359-69 (1986) BindingDB Entry DOI: 10.7270/Q24X58C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477933 (CHEMBL253041) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50007693 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1A receptor using in rat hippocampal tissue using [3H]-8-OH-DPAT as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476941 (CHEMBL233363) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477524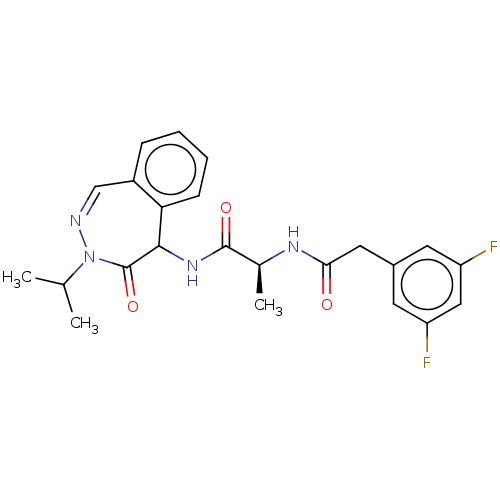 (CHEMBL246784) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50019368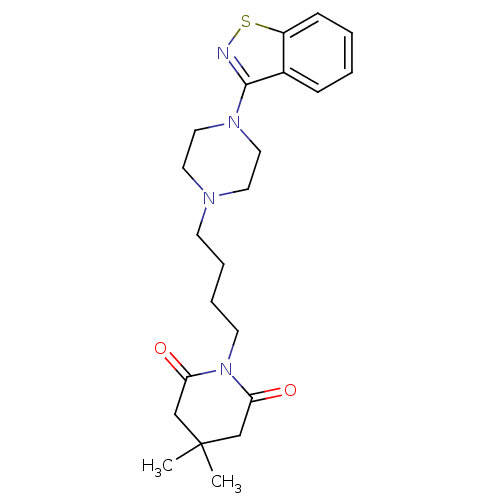 (1-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro affinity for cortical alpha-1 adrenergic receptor labelled with [3H]WB-4101 | J Med Chem 29: 359-69 (1986) BindingDB Entry DOI: 10.7270/Q24X58C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50007691 (3-Oxo-2-oxa-spiro[4.4]nonane-1-carboxylic acid [4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against rat 5-hydroxytryptamine 2 receptor in rat cerebral cortex tissue using [3H]-spiperone as radioligand | J Med Chem 34: 3316-28 (1991) BindingDB Entry DOI: 10.7270/Q29Z93VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50132321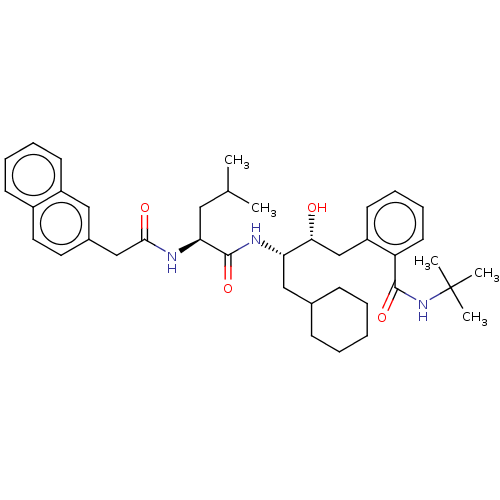 (CHEMBL298738) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma-secretase activity in H4 human neuroglioma cells by measuring amyloid beta production | Bioorg Med Chem Lett 14: 1917-21 (2004) BindingDB Entry DOI: 10.7270/Q2TT4T4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477943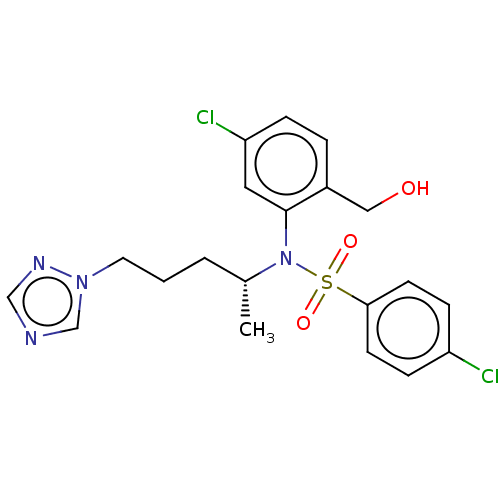 (CHEMBL437405) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in H4 cells assessed as reduction of amyloid beta-40 levels | Bioorg Med Chem Lett 18: 175-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.105 BindingDB Entry DOI: 10.7270/Q2K0772B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50476940 (CHEMBL233362) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of gamma secretase-mediated beta-APP cleavage in human H4 cells assessed by measuring Abeta40 production by ELISA | Bioorg Med Chem Lett 17: 4432-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.022 BindingDB Entry DOI: 10.7270/Q2708465 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 318 total ) | Next | Last >> |