

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human FGFR1 | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM50065454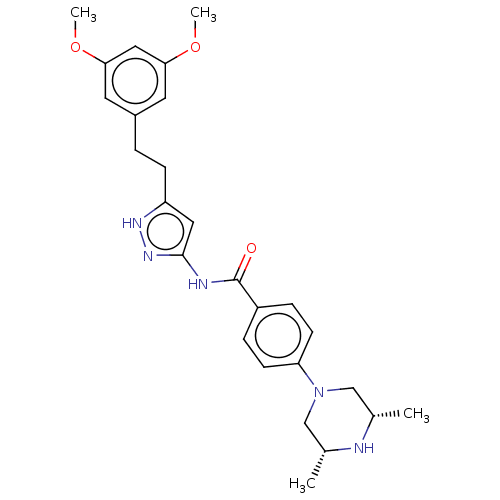 (CHEBI:63453 | CHEMBL3348846) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai, 201203, China; State Key Lab of New Drug& Pharmaceutical Process, Shanghai Key Lab of Anti-Infectives, State Ins Curated by ChEMBL | Assay Description Inhibition of FGFR1 (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 1 hr by ELISA | Eur J Med Chem 135: 370-381 (2017) Article DOI: 10.1016/j.ejmech.2017.04.039 BindingDB Entry DOI: 10.7270/Q27P91W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai, 201203, China; State Key Lab of New Drug& Pharmaceutical Process, Shanghai Key Lab of Anti-Infectives, State Ins Curated by ChEMBL | Assay Description Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 1 hr by ELISA | Eur J Med Chem 135: 370-381 (2017) Article DOI: 10.1016/j.ejmech.2017.04.039 BindingDB Entry DOI: 10.7270/Q27P91W5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human KDR | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai, 201203, China; State Key Lab of New Drug& Pharmaceutical Process, Shanghai Key Lab of Anti-Infectives, State Ins Curated by ChEMBL | Assay Description Inhibition of KDR (unknown origin) using poly (Glu, Tyr) 4:1 as substrate at after 1 hr by ELISA | Eur J Med Chem 135: 370-381 (2017) Article DOI: 10.1016/j.ejmech.2017.04.039 BindingDB Entry DOI: 10.7270/Q27P91W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50021574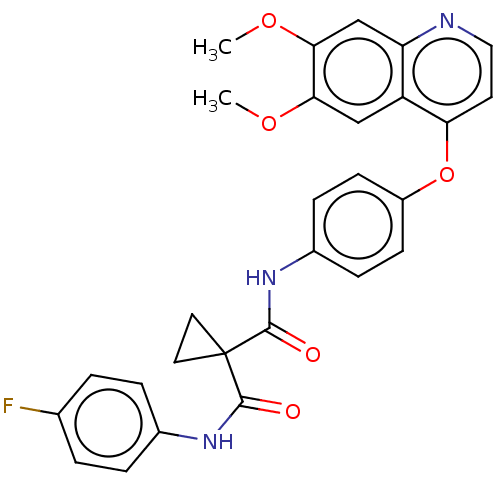 (BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai, 201203, China; State Key Lab of New Drug& Pharmaceutical Process, Shanghai Key Lab of Anti-Infectives, State Ins Curated by ChEMBL | Assay Description Inhibition of Ret (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 1 hr by ELISA | Eur J Med Chem 135: 370-381 (2017) Article DOI: 10.1016/j.ejmech.2017.04.039 BindingDB Entry DOI: 10.7270/Q27P91W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50021574 (BMS-907351 | CABOZANTINIB | CHEBI:72317 | Cabomety...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai, 201203, China; State Key Lab of New Drug& Pharmaceutical Process, Shanghai Key Lab of Anti-Infectives, State Ins Curated by ChEMBL | Assay Description Inhibition of AXL (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 1 hr by ELISA | Eur J Med Chem 135: 370-381 (2017) Article DOI: 10.1016/j.ejmech.2017.04.039 BindingDB Entry DOI: 10.7270/Q27P91W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267625 (CHEMBL4077172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai, 201203, China; State Key Lab of New Drug& Pharmaceutical Process, Shanghai Key Lab of Anti-Infectives, State Ins Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) | Eur J Med Chem 135: 370-381 (2017) Article DOI: 10.1016/j.ejmech.2017.04.039 BindingDB Entry DOI: 10.7270/Q27P91W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50306682 ((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai, 201203, China; State Key Lab of New Drug& Pharmaceutical Process, Shanghai Key Lab of Anti-Infectives, State Ins Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 1 hr by ELISA | Eur J Med Chem 135: 370-381 (2017) Article DOI: 10.1016/j.ejmech.2017.04.039 BindingDB Entry DOI: 10.7270/Q27P91W5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267626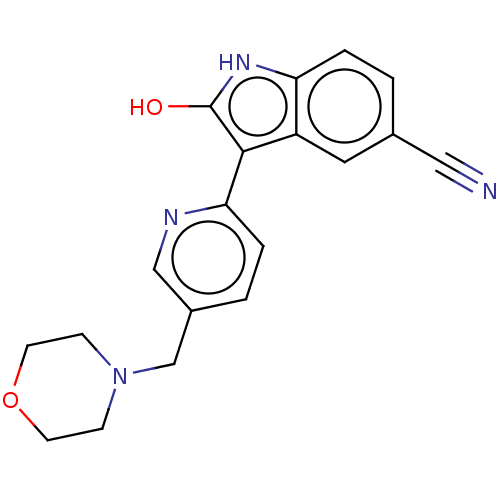 (AZ-11548415 | AZD-1080) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, Shanghai, 201203, China; State Key Lab of New Drug& Pharmaceutical Process, Shanghai Key Lab of Anti-Infectives, State Ins Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) | Eur J Med Chem 135: 370-381 (2017) Article DOI: 10.1016/j.ejmech.2017.04.039 BindingDB Entry DOI: 10.7270/Q27P91W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM7781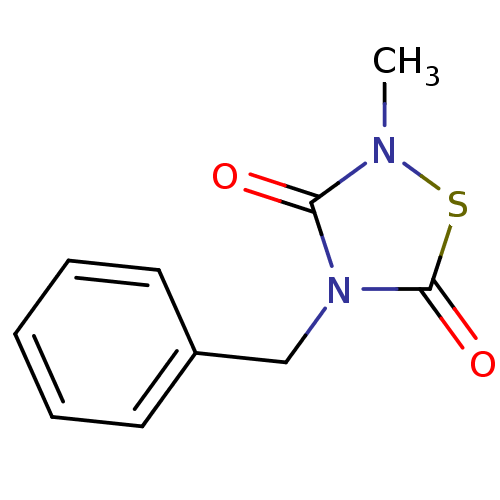 (4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using 650HSSPHQ(pS)EDEEE as substrate after 30 mins by luminescence assay | Eur J Med Chem 61: 95-103 (2013) Article DOI: 10.1016/j.ejmech.2012.09.021 BindingDB Entry DOI: 10.7270/Q24J0GF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM7781 (4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of GSK3beta by kinase-glo assay method | Bioorg Med Chem Lett 22: 7232-6 (2012) Article DOI: 10.1016/j.bmcl.2012.09.043 BindingDB Entry DOI: 10.7270/Q2KD2034 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254217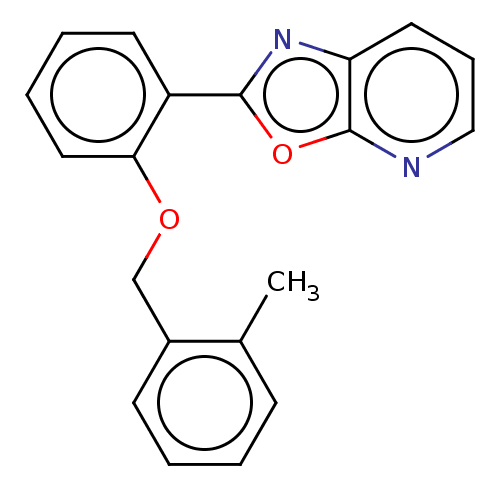 (CHEMBL4092416) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117841 (CHEMBL3613983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254231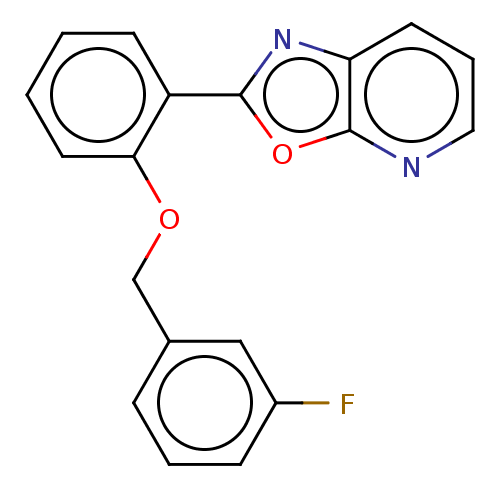 (CHEMBL4084661) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254224 (CHEMBL4100851) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254232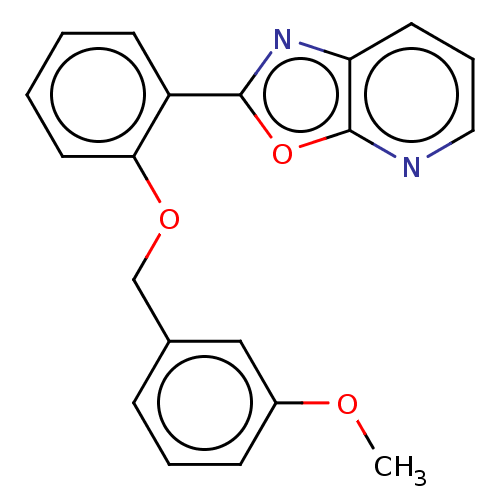 (CHEMBL4076807) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254233 (CHEMBL4077085) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254229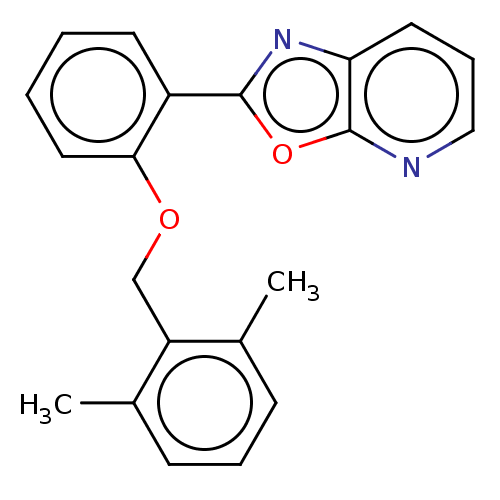 (CHEMBL4092156) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50401965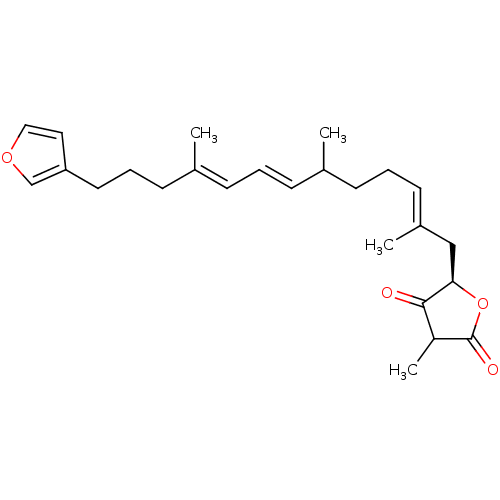 (PALINURIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Non-ATP competitive inhibition of GSK3beta | Bioorg Med Chem Lett 22: 7232-6 (2012) Article DOI: 10.1016/j.bmcl.2012.09.043 BindingDB Entry DOI: 10.7270/Q2KD2034 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50401965 (PALINURIN) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of GSK3beta (unknown origin) | Eur J Med Chem 61: 95-103 (2013) Article DOI: 10.1016/j.ejmech.2012.09.021 BindingDB Entry DOI: 10.7270/Q24J0GF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254223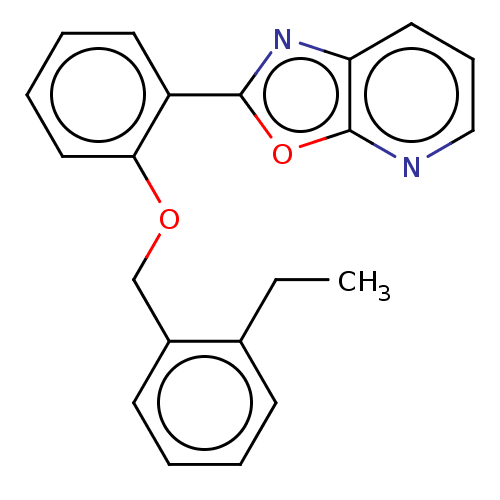 (CHEMBL4077313) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117832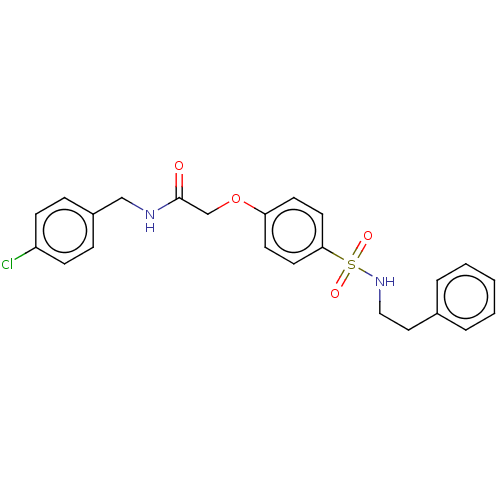 (CHEMBL3613975) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117840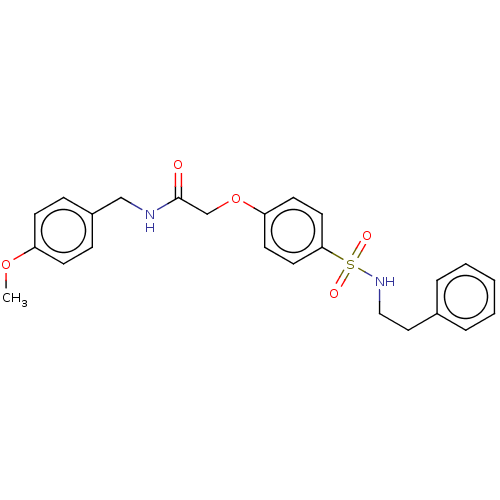 (CHEMBL3612087) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117845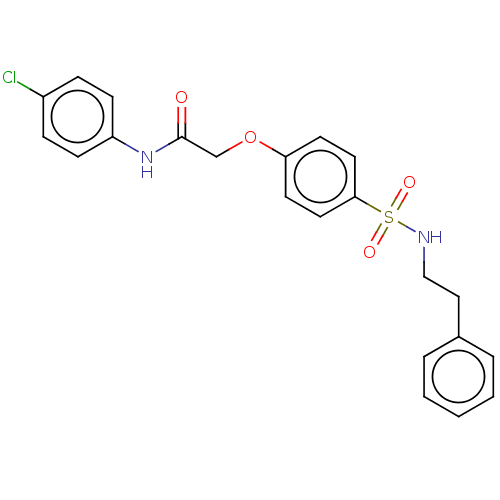 (CHEMBL3613987) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117842 (CHEMBL3613984) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117853 (CHEMBL3613995) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50232478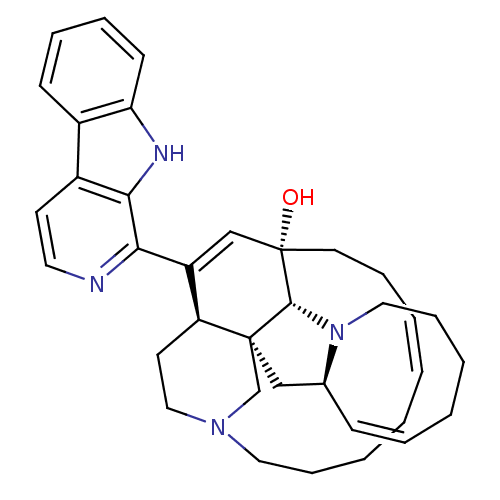 (CHEMBL403233) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Non-ATP competitive inhibition of GSK3beta | Bioorg Med Chem Lett 22: 7232-6 (2012) Article DOI: 10.1016/j.bmcl.2012.09.043 BindingDB Entry DOI: 10.7270/Q2KD2034 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117846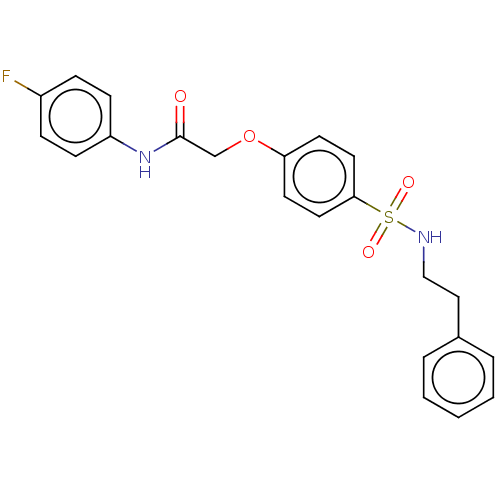 (CHEMBL3613988) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117848 (CHEMBL3613990) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117844 (CHEMBL3613986) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117843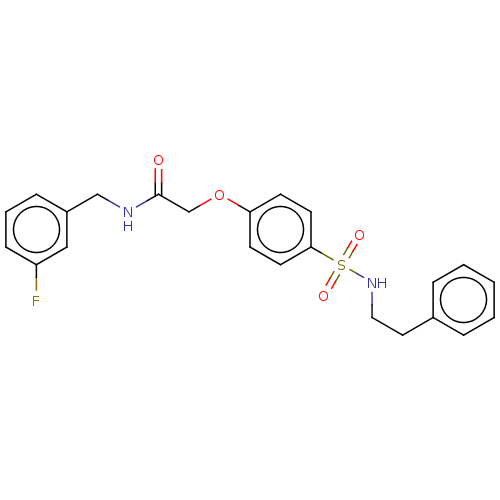 (CHEMBL3613985) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254230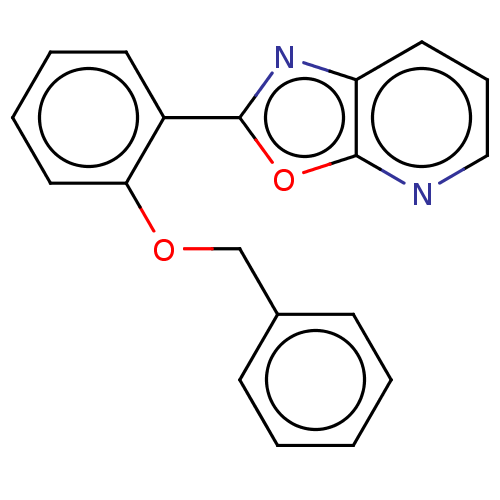 (CHEMBL4093733) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469285 (CHEMBL4279567) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469279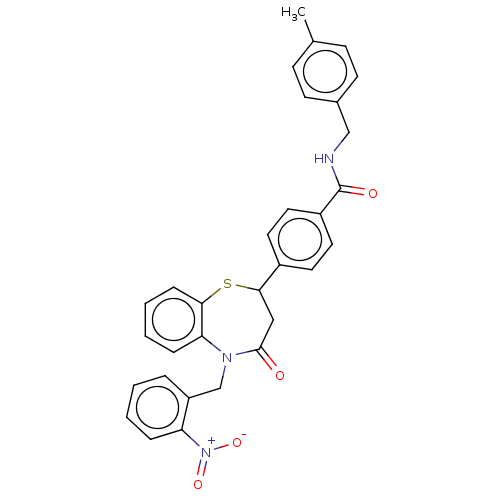 (CHEMBL4283032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469288 (CHEMBL4290418) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469283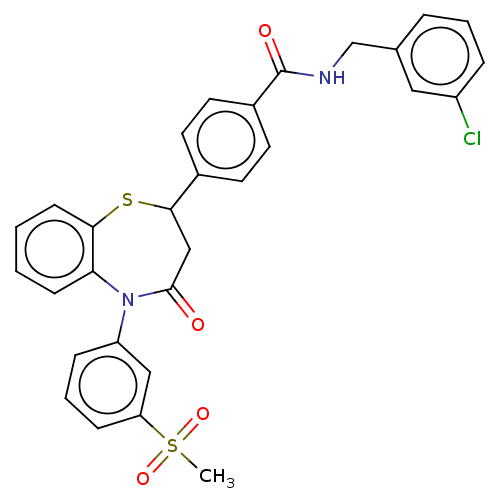 (CHEMBL4284111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254221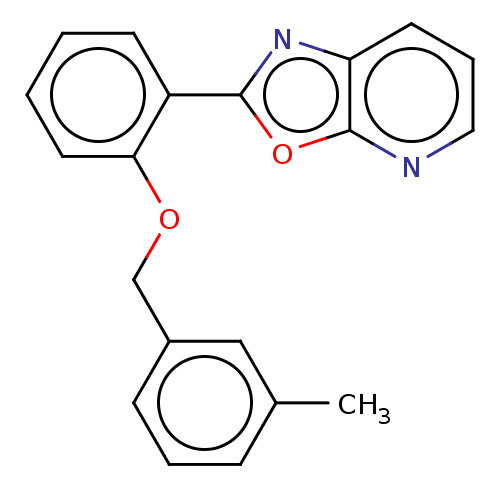 (CHEMBL4093369) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469289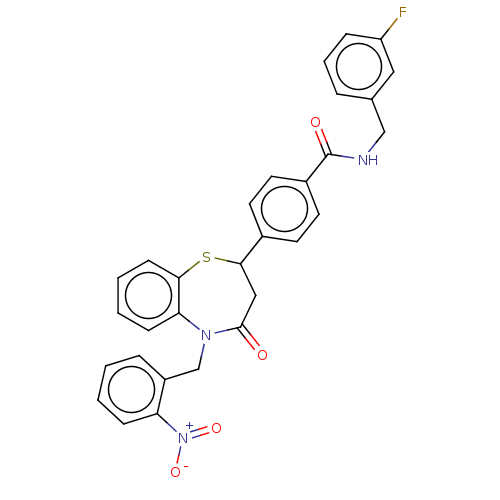 (CHEMBL4293500) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50254222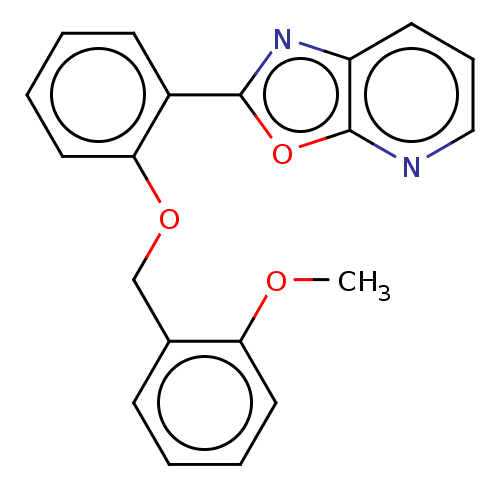 (CHEMBL4099898) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469306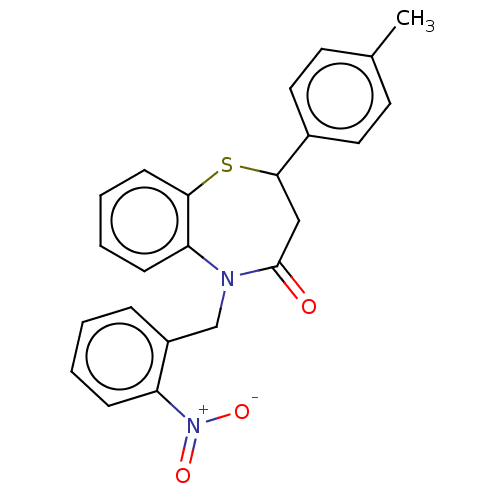 (CHEMBL4278749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469281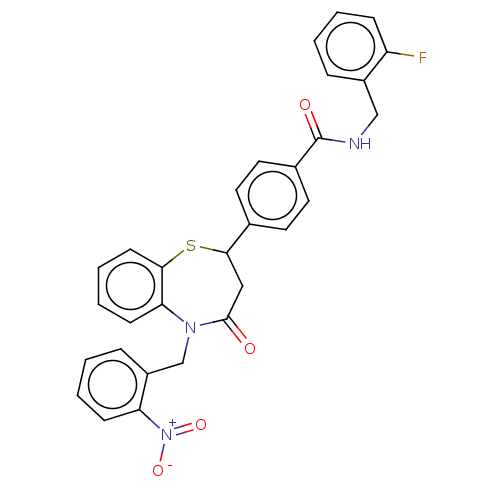 (CHEMBL4287006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469290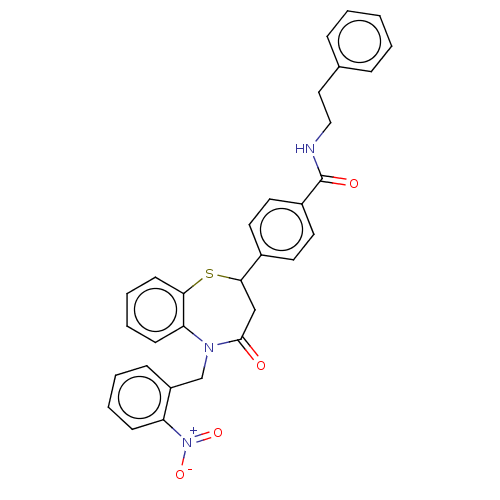 (CHEMBL4287420) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 2 (Homo sapiens (Human)) | BDBM50447012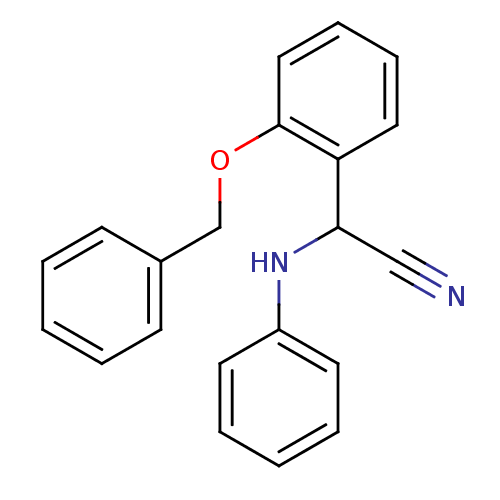 (CHEMBL1446389) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826, Zhangheng Rd., Shanghai 201203, China. Curated by ChEMBL | Assay Description Inhibition of purified SMS2 (unknown origin) pre-incubated for 5 mins followed by DMPC and C6-NBD-ceramide addition and measured after 30 mins by HPL... | Bioorg Med Chem Lett 27: 3511-3515 (2017) Article DOI: 10.1016/j.bmcl.2017.05.074 BindingDB Entry DOI: 10.7270/Q28918BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469295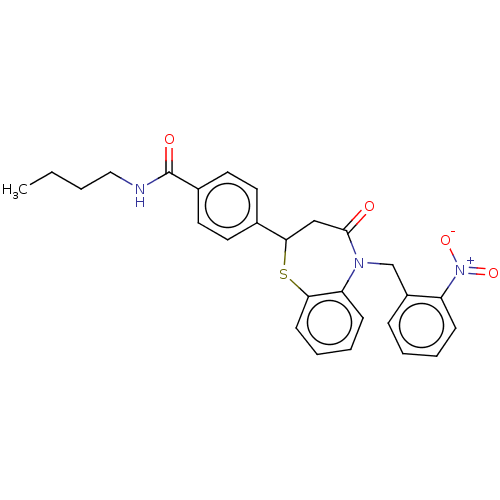 (CHEMBL4282621) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50428463 (CHEMBL2334795) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using 650HSSPHQ(pS)EDEEE as substrate after 30 mins by luminescence assay | Eur J Med Chem 61: 95-103 (2013) Article DOI: 10.1016/j.ejmech.2012.09.021 BindingDB Entry DOI: 10.7270/Q24J0GF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylcholine:ceramide cholinephosphotransferase 1 (Homo sapiens (Human)) | BDBM50117839 (CHEMBL3613982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of sphingomyelin synthase-1 (unknown origin) expressed in HeLa cells using C6-NBD-Cer and DMPC as substrate after 2 hrs by fluorescent HPL... | Bioorg Med Chem 23: 6173-84 (2015) Article DOI: 10.1016/j.bmc.2015.07.060 BindingDB Entry DOI: 10.7270/Q2NG4SF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50401955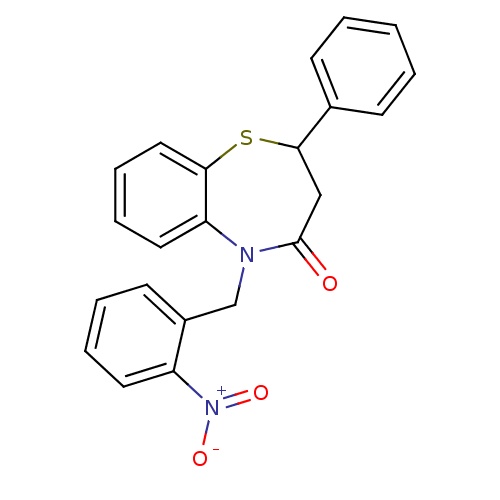 (CHEMBL2207940) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta using 650HSSPHQ(pS)EDEEE as substrate after 30 mins by luminescence assay | Eur J Med Chem 61: 95-103 (2013) Article DOI: 10.1016/j.ejmech.2012.09.021 BindingDB Entry DOI: 10.7270/Q24J0GF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50469287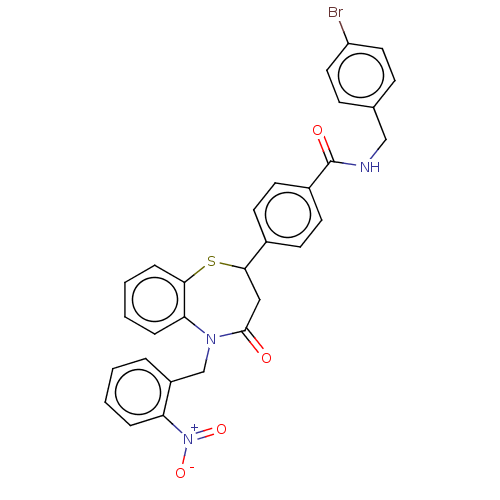 (CHEMBL4294290) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged human GSK-3beta expressed in Baculovirus infected SF9 cells using prephosphorylated-GS2 polype... | Bioorg Med Chem 26: 5479-5493 (2018) Article DOI: 10.1016/j.bmc.2018.09.027 BindingDB Entry DOI: 10.7270/Q2319ZMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50401955 (CHEMBL2207940) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of GSK3beta by kinase-glo assay method | Bioorg Med Chem Lett 22: 7232-6 (2012) Article DOI: 10.1016/j.bmcl.2012.09.043 BindingDB Entry DOI: 10.7270/Q2KD2034 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 168 total ) | Next | Last >> |