

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543457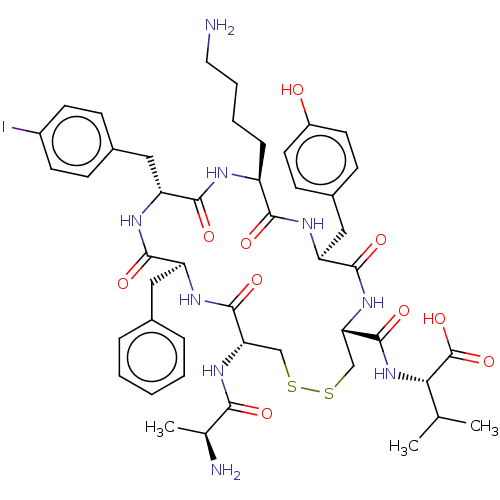 (CHEMBL4635039) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543458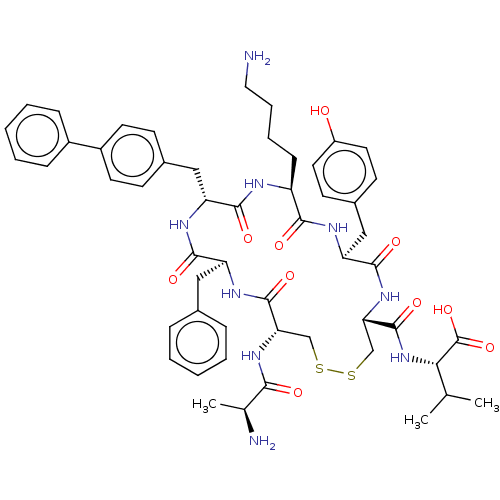 (CHEMBL4642307) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459265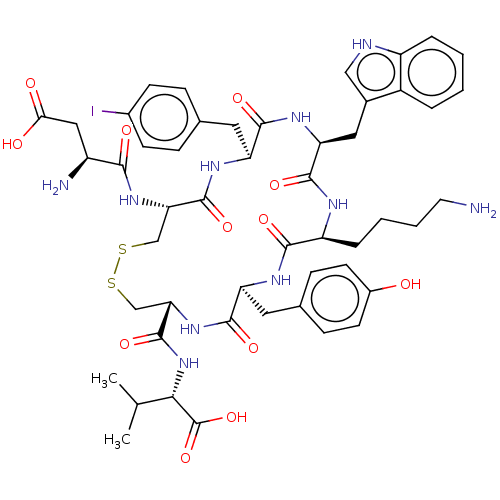 (CHEMBL4205274) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445383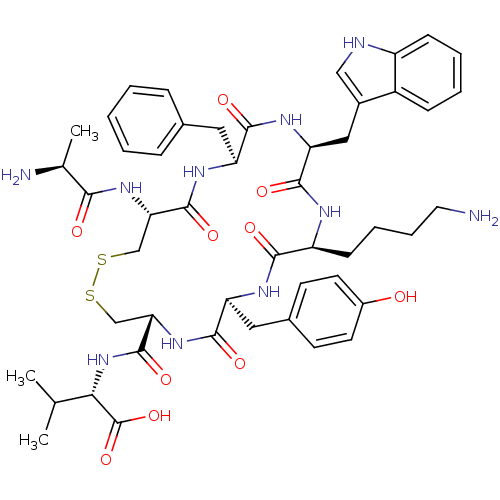 (CHEMBL3104471) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459261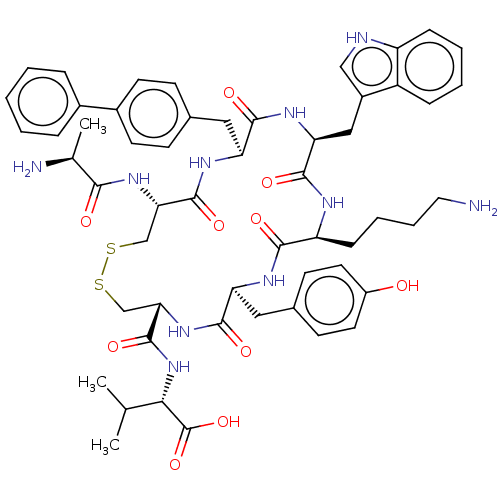 (CHEMBL4208184) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459266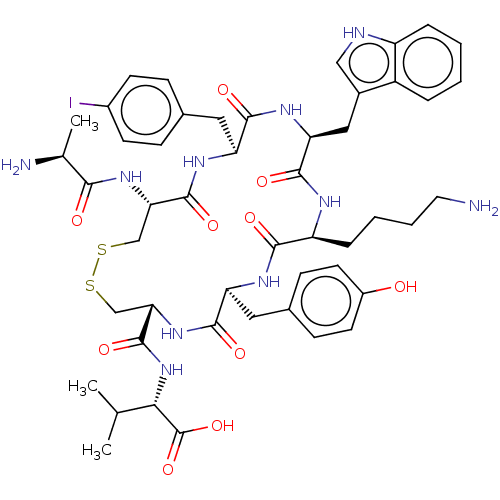 (CHEMBL4217514) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543461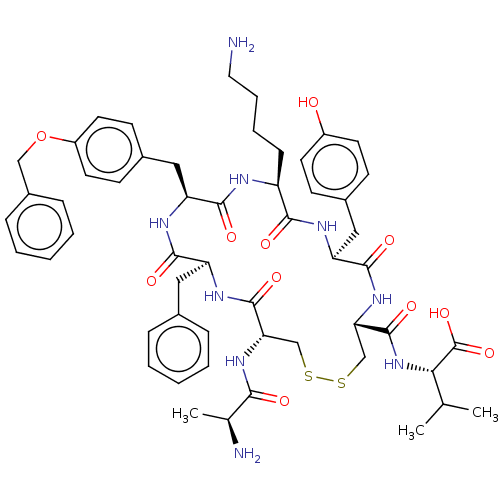 (CHEMBL4638346) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543459 (CHEMBL4643905) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50445383 (CHEMBL3104471) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459268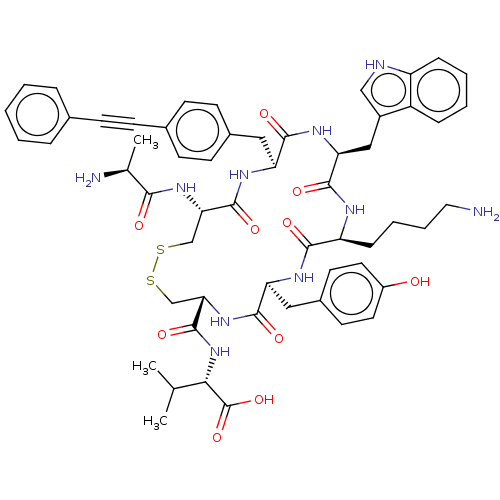 (CHEMBL4213101) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543462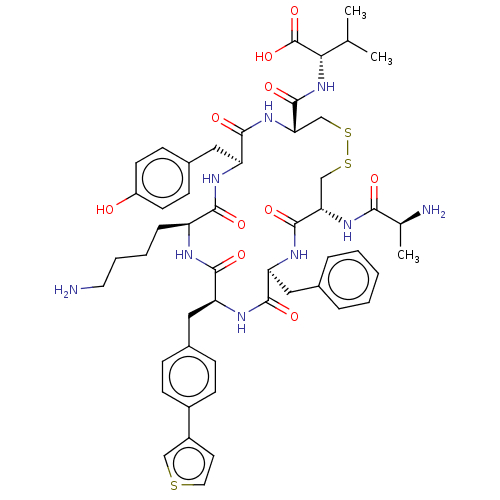 (CHEMBL4633010) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543463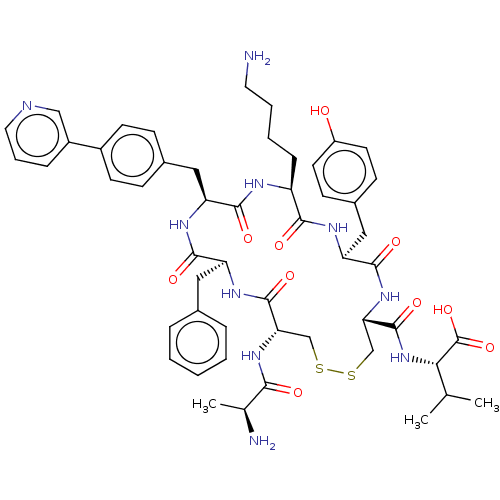 (CHEMBL4638745) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543460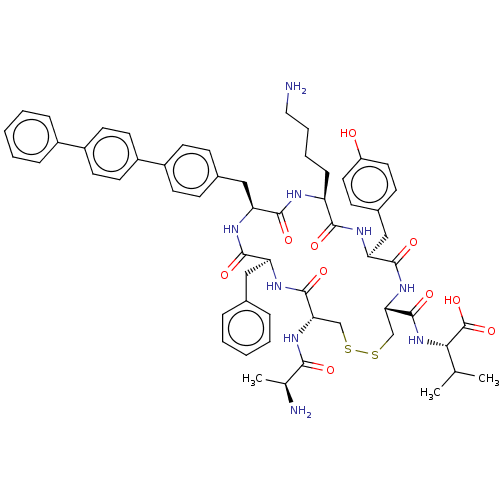 (CHEMBL4642313) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50543464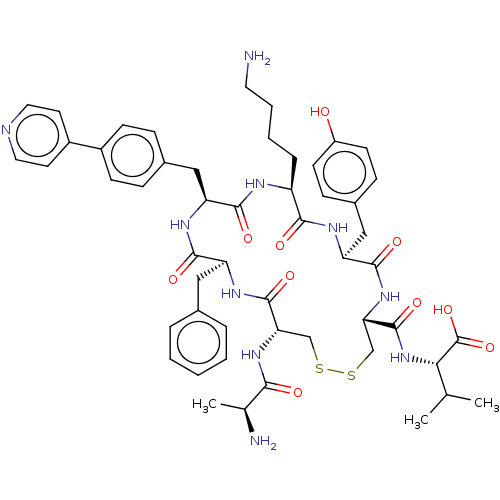 (CHEMBL4635150) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Antagonist activity at human UT receptor expressed in HEK293 cells using Na [125I] incubated for 2 hrs by gamma counting method | ACS Med Chem Lett 11: 1717-1722 (2020) Article DOI: 10.1021/acsmedchemlett.0c00223 BindingDB Entry DOI: 10.7270/Q269775C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459267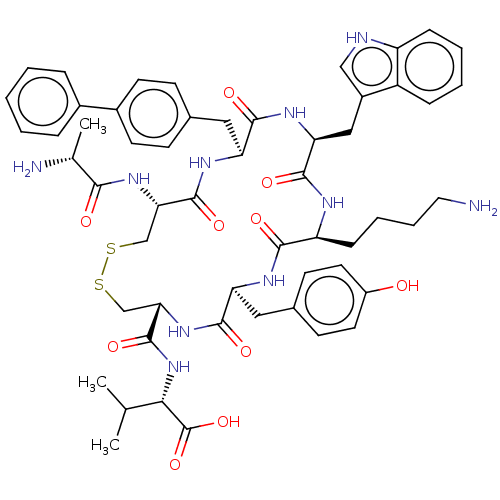 (CHEMBL4209654) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459262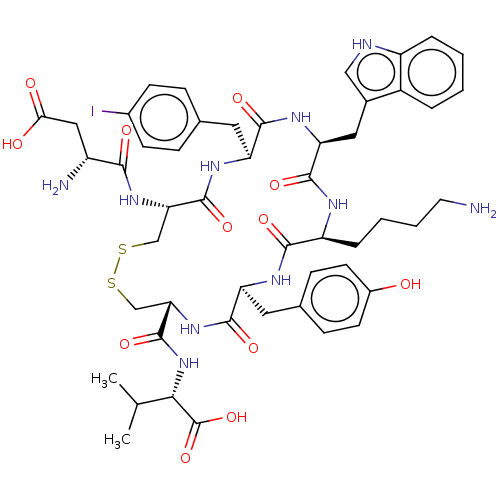 (CHEMBL4213538) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459263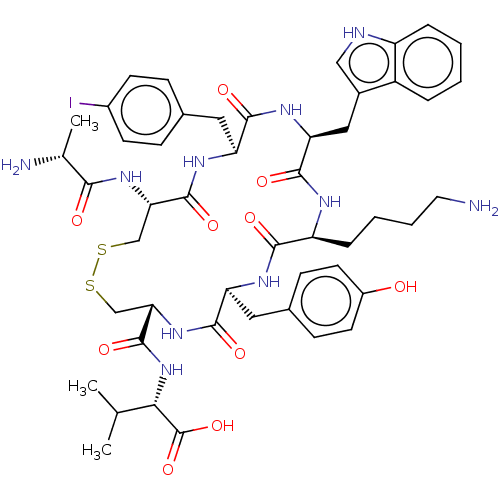 (CHEMBL4204786) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50459264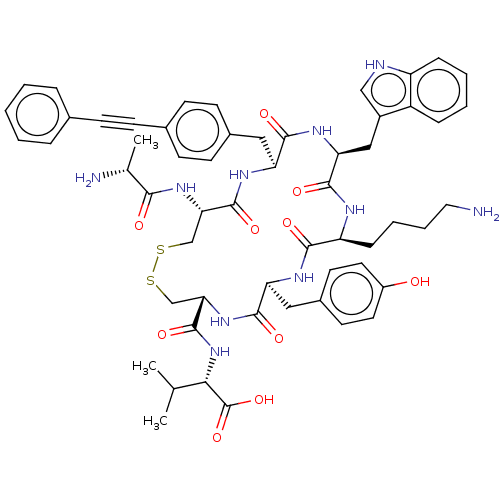 (CHEMBL4216988) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ du Qu£bec Curated by ChEMBL | Assay Description Displacement of [Na125I]-synthetic URP from human UT receptor expressed in HEK293 cell membranes incubated for 2 hrs by gamma-counting | J Med Chem 61: 8707-8716 (2018) Article DOI: 10.1021/acs.jmedchem.8b00789 BindingDB Entry DOI: 10.7270/Q2959M57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269944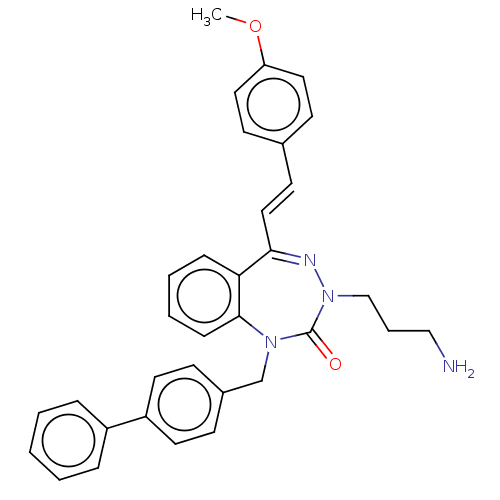 (CHEMBL4081970) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269945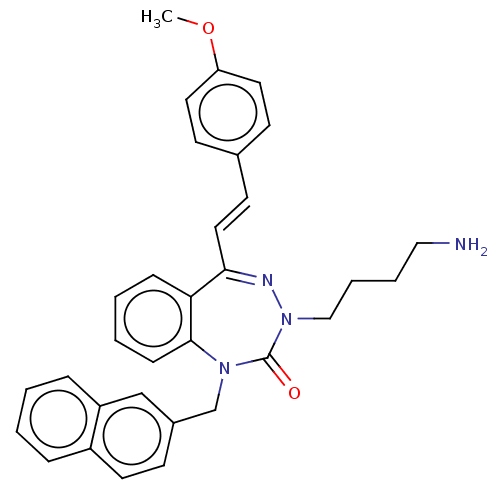 (CHEMBL4092221) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269946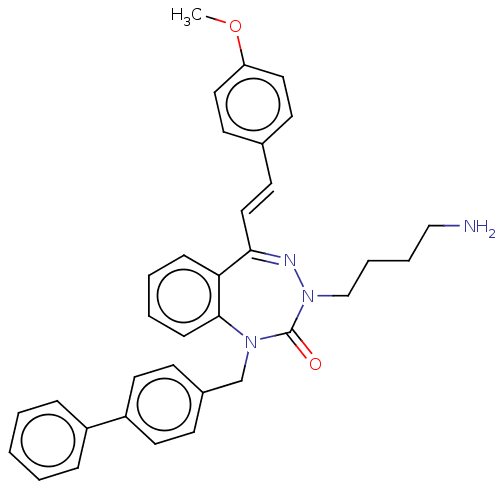 (CHEMBL4071437) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269947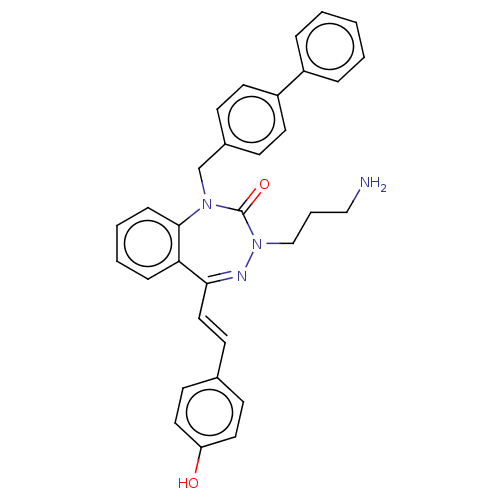 (CHEMBL4062799) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269949 (CHEMBL4074175) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269950 (CHEMBL4100932) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269951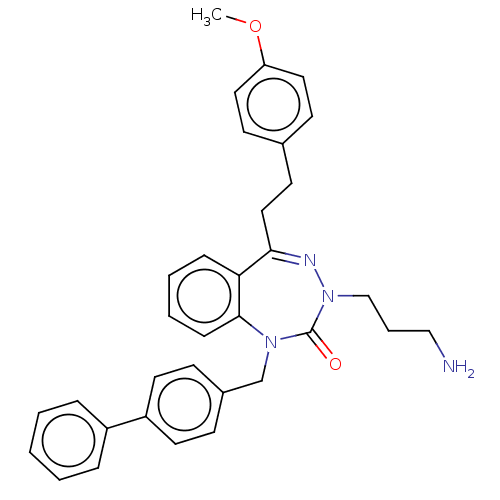 (CHEMBL4069649) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269952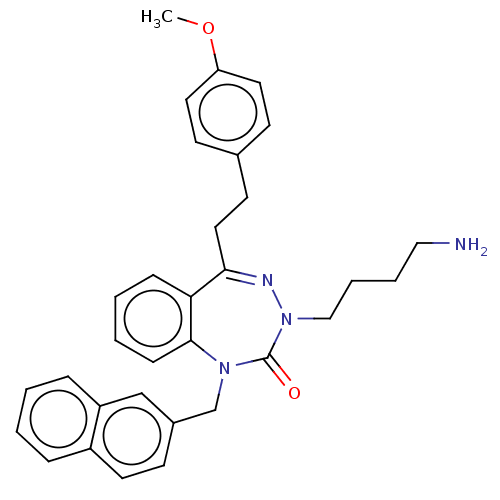 (CHEMBL4085539) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269953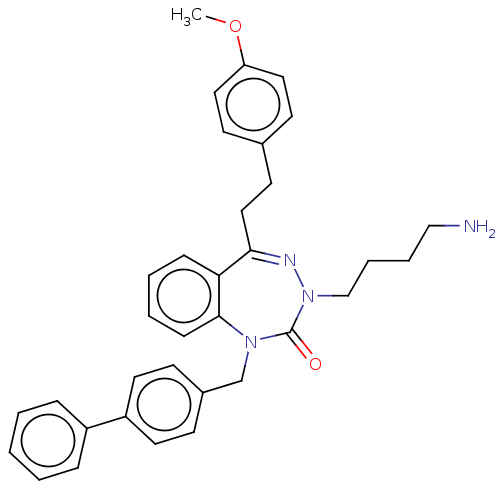 (CHEMBL4103468) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269954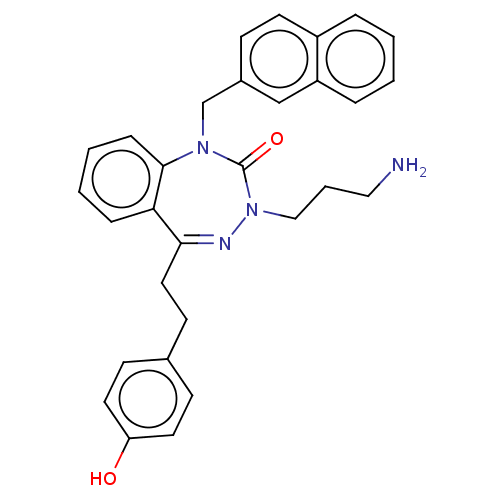 (CHEMBL4077689) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269955 (CHEMBL4066696) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269956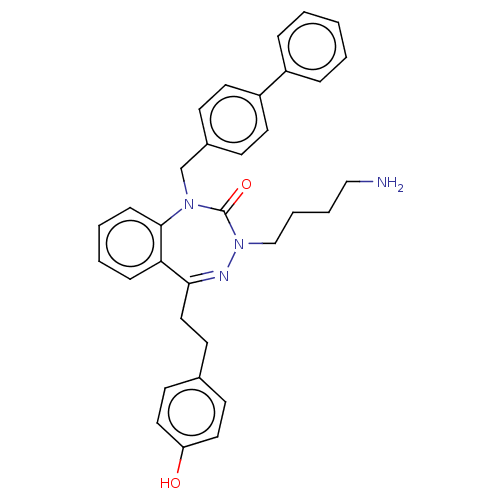 (CHEMBL4087993) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269957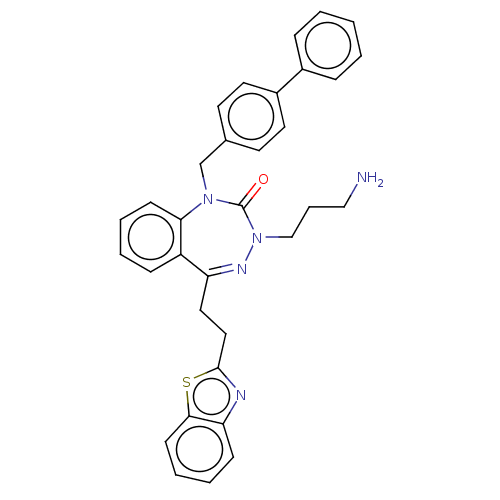 (CHEMBL4068478) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269958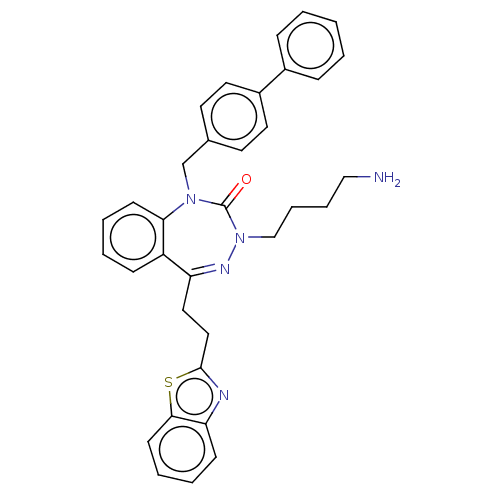 (CHEMBL4076985) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269959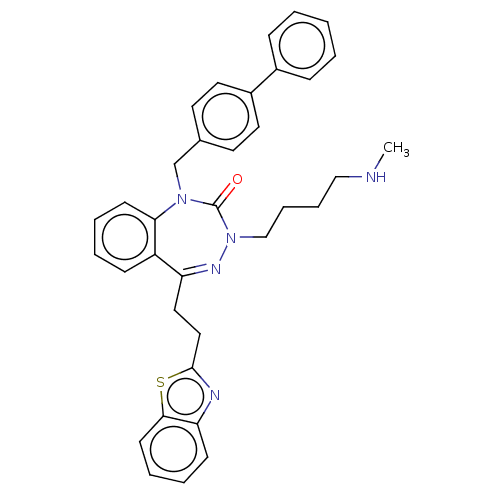 (CHEMBL4064003) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269960 (CHEMBL4076150) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269964 (CHEMBL4077479) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269967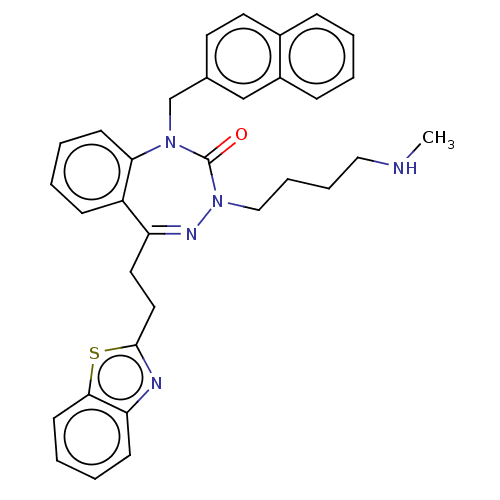 (CHEMBL4096184) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269968 (CHEMBL4087877) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269969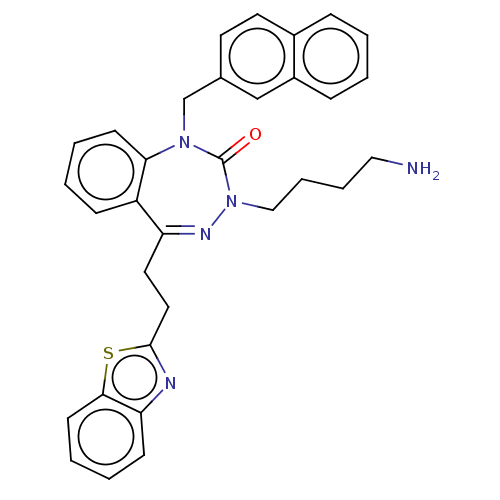 (CHEMBL4060368) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in human urotensin-2-mediated aortic ring contr... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269944 (CHEMBL4081970) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269945 (CHEMBL4092221) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269946 (CHEMBL4071437) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269947 (CHEMBL4062799) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269949 (CHEMBL4074175) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269950 (CHEMBL4100932) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269970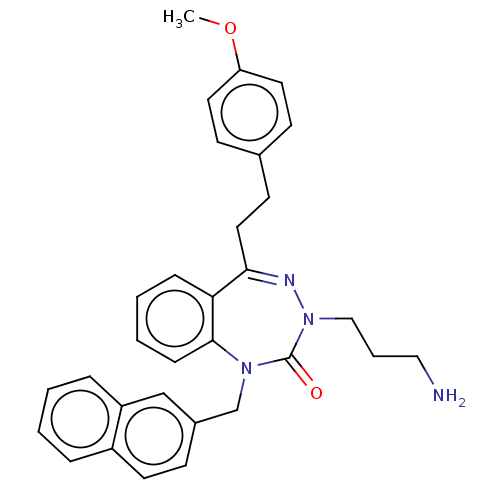 (CHEMBL4065552) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269953 (CHEMBL4103468) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269954 (CHEMBL4077689) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269980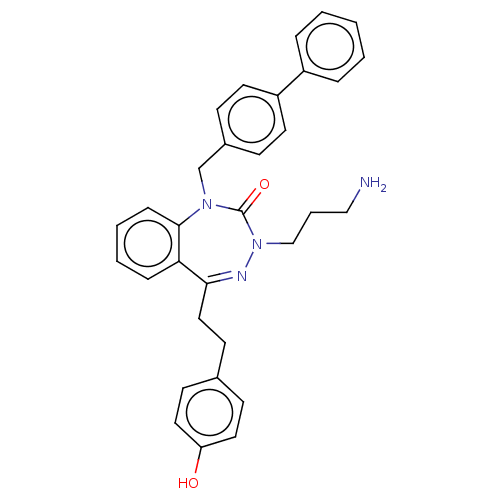 (CHEMBL4095707) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269955 (CHEMBL4066696) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50269957 (CHEMBL4068478) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Allosteric modulation of urotensin-2 receptor in Sprague-Dawley rat thoracic aorta assessed as change in URP-mediated aortic ring contraction by meas... | J Med Chem 60: 9838-9859 (2017) Article DOI: 10.1021/acs.jmedchem.7b01525 BindingDB Entry DOI: 10.7270/Q2V1279D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 138 total ) | Next | Last >> |