 Found 22 hits with Last Name = 'casanova' and Initial = 'e'
Found 22 hits with Last Name = 'casanova' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194179
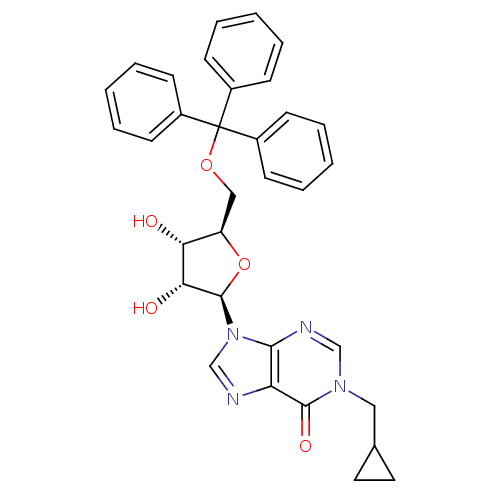
(1-(cyclopropyl)methyl-5'-O-tritylinosine | CHEMBL3...)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2c1ncn(CC1CC1)c2=O Show InChI InChI=1S/C33H32N4O5/c38-28-26(42-32(29(28)39)37-21-34-27-30(37)35-20-36(31(27)40)18-22-16-17-22)19-41-33(23-10-4-1-5-11-23,24-12-6-2-7-13-24)25-14-8-3-9-15-25/h1-15,20-22,26,28-29,32,38-39H,16-19H2/t26-,28-,29-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194181
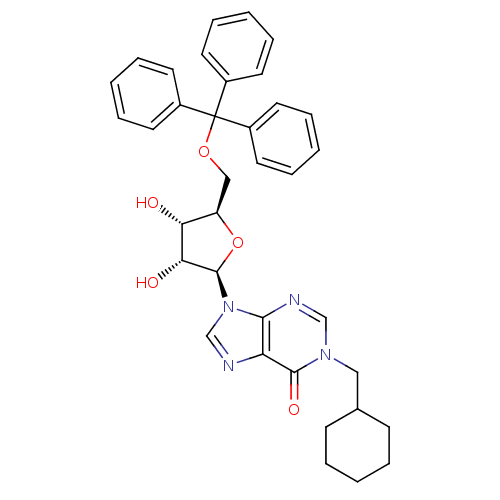
(1-(cyclohexyl)methyl-5'-O-tritylinosine | CHEMBL21...)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2c1ncn(CC1CCCCC1)c2=O Show InChI InChI=1S/C36H38N4O5/c41-31-29(22-44-36(26-15-7-2-8-16-26,27-17-9-3-10-18-27)28-19-11-4-12-20-28)45-35(32(31)42)40-24-37-30-33(40)38-23-39(34(30)43)21-25-13-5-1-6-14-25/h2-4,7-12,15-20,23-25,29,31-32,35,41-42H,1,5-6,13-14,21-22H2/t29-,31-,32-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194173
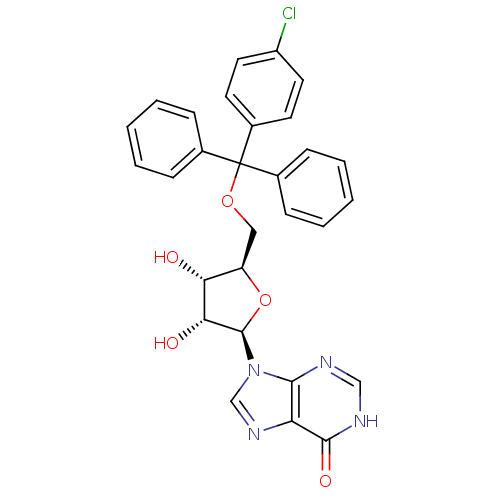
(5'-O-[(4-chlorophenyl)-1,1-(diphenyl)methyl]inosin...)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccc(Cl)cc2)O[C@H]([C@@H]1O)n1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C29H25ClN4O5/c30-21-13-11-20(12-14-21)29(18-7-3-1-4-8-18,19-9-5-2-6-10-19)38-15-22-24(35)25(36)28(39-22)34-17-33-23-26(34)31-16-32-27(23)37/h1-14,16-17,22,24-25,28,35-36H,15H2,(H,31,32,37)/t22-,24-,25-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194174

(1-benzyl-5'-O-tritylinosine | CHEMBL441540)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2c1ncn(Cc1ccccc1)c2=O Show InChI InChI=1S/C36H32N4O5/c41-31-29(22-44-36(26-15-7-2-8-16-26,27-17-9-3-10-18-27)28-19-11-4-12-20-28)45-35(32(31)42)40-24-37-30-33(40)38-23-39(34(30)43)21-25-13-5-1-6-14-25/h1-20,23-24,29,31-32,35,41-42H,21-22H2/t29-,31-,32-,35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194175

(1-allyl-5'-O-tritylinosine | CHEMBL213891)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2c1ncn(CC=C)c2=O Show InChI InChI=1S/C32H30N4O5/c1-2-18-35-20-34-29-26(30(35)39)33-21-36(29)31-28(38)27(37)25(41-31)19-40-32(22-12-6-3-7-13-22,23-14-8-4-9-15-23)24-16-10-5-11-17-24/h2-17,20-21,25,27-28,31,37-38H,1,18-19H2/t25-,27-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194170
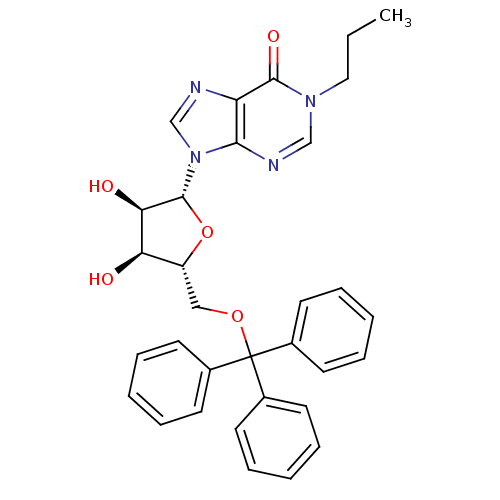
(1-propyl-5'-O-tritylinosine | CHEMBL213890)Show SMILES CCCn1cnc2n(cnc2c1=O)[C@@H]1O[C@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)[C@@H](O)[C@H]1O Show InChI InChI=1S/C32H32N4O5/c1-2-18-35-20-34-29-26(30(35)39)33-21-36(29)31-28(38)27(37)25(41-31)19-40-32(22-12-6-3-7-13-22,23-14-8-4-9-15-23)24-16-10-5-11-17-24/h3-17,20-21,25,27-28,31,37-38H,2,18-19H2,1H3/t25-,27-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194176
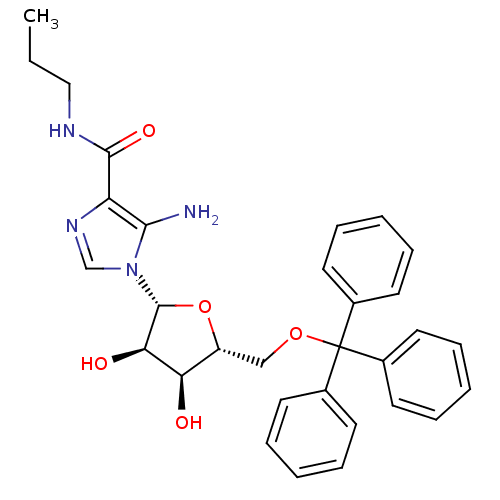
(5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...)Show SMILES CCCNC(=O)c1ncn([C@@H]2O[C@H](COC(c3ccccc3)(c3ccccc3)c3ccccc3)[C@@H](O)[C@H]2O)c1N Show InChI InChI=1S/C31H34N4O5/c1-2-18-33-29(38)25-28(32)35(20-34-25)30-27(37)26(36)24(40-30)19-39-31(21-12-6-3-7-13-21,22-14-8-4-9-15-22)23-16-10-5-11-17-23/h3-17,20,24,26-27,30,36-37H,2,18-19,32H2,1H3,(H,33,38)/t24-,26-,27-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194168

(9-((2R,3R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)m...)Show SMILES COc1ccc(cc1)C(OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c1nc[nH]c2=O)(c1ccccc1)c1ccc(OC)cc1 Show InChI InChI=1S/C31H30N4O7/c1-39-22-12-8-20(9-13-22)31(19-6-4-3-5-7-19,21-10-14-23(40-2)15-11-21)41-16-24-26(36)27(37)30(42-24)35-18-34-25-28(35)32-17-33-29(25)38/h3-15,17-18,24,26-27,30,36-37H,16H2,1-2H3,(H,32,33,38)/t24-,26-,27-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194184

(5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...)Show SMILES Nc1c(ncn1[C@@H]1O[C@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)[C@@H](O)[C@H]1O)C(=O)NCC1CC1 Show InChI InChI=1S/C32H34N4O5/c33-29-26(30(39)34-18-21-16-17-21)35-20-36(29)31-28(38)27(37)25(41-31)19-40-32(22-10-4-1-5-11-22,23-12-6-2-7-13-23)24-14-8-3-9-15-24/h1-15,20-21,25,27-28,31,37-38H,16-19,33H2,(H,34,39)/t25-,27-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194186

(2',3'-Di-O-acetyl-5'-O-trityl-1-[(2-methoxyethoxy)...)Show SMILES COCCOCn1cnc2n(cnc2c1=O)[C@@H]1O[C@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)[C@@H](OC(C)=O)[C@H]1OC(C)=O Show InChI InChI=1S/C37H38N4O9/c1-25(42)48-32-30(21-47-37(27-13-7-4-8-14-27,28-15-9-5-10-16-28)29-17-11-6-12-18-29)50-36(33(32)49-26(2)43)41-23-38-31-34(41)39-22-40(35(31)44)24-46-20-19-45-3/h4-18,22-23,30,32-33,36H,19-21,24H2,1-3H3/t30-,32-,33-,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194185

(5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...)Show SMILES Nc1c(ncn1[C@@H]1O[C@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)[C@@H](O)[C@H]1O)C(=O)NCC=C Show InChI InChI=1S/C31H32N4O5/c1-2-18-33-29(38)25-28(32)35(20-34-25)30-27(37)26(36)24(40-30)19-39-31(21-12-6-3-7-13-21,22-14-8-4-9-15-22)23-16-10-5-11-17-23/h2-17,20,24,26-27,30,36-37H,1,18-19,32H2,(H,33,38)/t24-,26-,27-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194180

(1-[(E)-3-methoxycarbonyl-2-propenyl]-5'-O-tritylin...)Show SMILES COC(=O)\C=C\Cn1cnc2n(cnc2c1=O)[C@@H]1O[C@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)[C@@H](O)[C@H]1O Show InChI InChI=1S/C34H32N4O7/c1-43-27(39)18-11-19-37-21-36-31-28(32(37)42)35-22-38(31)33-30(41)29(40)26(45-33)20-44-34(23-12-5-2-6-13-23,24-14-7-3-8-15-24)25-16-9-4-10-17-25/h2-18,21-22,26,29-30,33,40-41H,19-20H2,1H3/b18-11+/t26-,29-,30-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194178

(6-chloro-9-(5-O-trityl-beta-D-ribofuranosyl)purine...)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2c(Cl)ncnc12 Show InChI InChI=1S/C29H25ClN4O4/c30-26-23-27(32-17-31-26)34(18-33-23)28-25(36)24(35)22(38-28)16-37-29(19-10-4-1-5-11-19,20-12-6-2-7-13-20)21-14-8-3-9-15-21/h1-15,17-18,22,24-25,28,35-36H,16H2/t22-,24-,25-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194172

(5'-O-tritylinosine | CHEMBL386148)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C29H26N4O5/c34-24-22(38-28(25(24)35)33-18-32-23-26(33)30-17-31-27(23)36)16-37-29(19-10-4-1-5-11-19,20-12-6-2-7-13-20)21-14-8-3-9-15-21/h1-15,17-18,22,24-25,28,34-35H,16H2,(H,30,31,36)/t22-,24-,25-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194189

(9-(5-O-trityl-beta-D-ribofuranosyl)purine | CHEMBL...)Show SMILES O[C@@H]1[C@@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)O[C@H]([C@@H]1O)n1cnc2cncnc12 Show InChI InChI=1S/C29H26N4O4/c34-25-24(37-28(26(25)35)33-19-32-23-16-30-18-31-27(23)33)17-36-29(20-10-4-1-5-11-20,21-12-6-2-7-13-21)22-14-8-3-9-15-22/h1-16,18-19,24-26,28,34-35H,17H2/t24-,25-,26-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194177

(1-methyl-5'-O-tritylinosine | CHEMBL212853)Show SMILES Cn1cnc2n(cnc2c1=O)[C@@H]1O[C@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)[C@@H](O)[C@H]1O Show InChI InChI=1S/C30H28N4O5/c1-33-18-32-27-24(28(33)37)31-19-34(27)29-26(36)25(35)23(39-29)17-38-30(20-11-5-2-6-12-20,21-13-7-3-8-14-21)22-15-9-4-10-16-22/h2-16,18-19,23,25-26,29,35-36H,17H2,1H3/t23-,25-,26-,29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194187
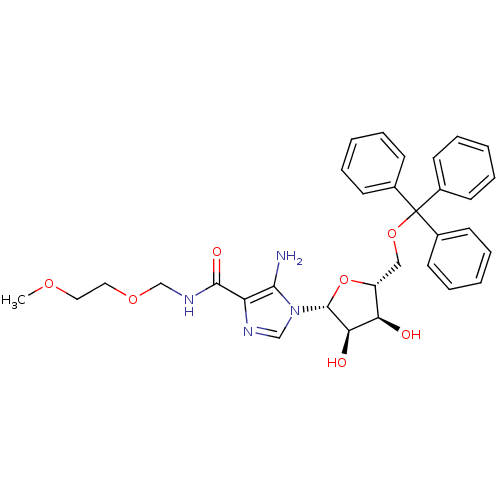
(5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...)Show SMILES COCCOCNC(=O)c1ncn([C@@H]2O[C@H](COC(c3ccccc3)(c3ccccc3)c3ccccc3)[C@@H](O)[C@H]2O)c1N Show InChI InChI=1S/C32H36N4O7/c1-40-17-18-41-21-35-30(39)26-29(33)36(20-34-26)31-28(38)27(37)25(43-31)19-42-32(22-11-5-2-6-12-22,23-13-7-3-8-14-23)24-15-9-4-10-16-24/h2-16,20,25,27-28,31,37-38H,17-19,21,33H2,1H3,(H,35,39)/t25-,27-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194188
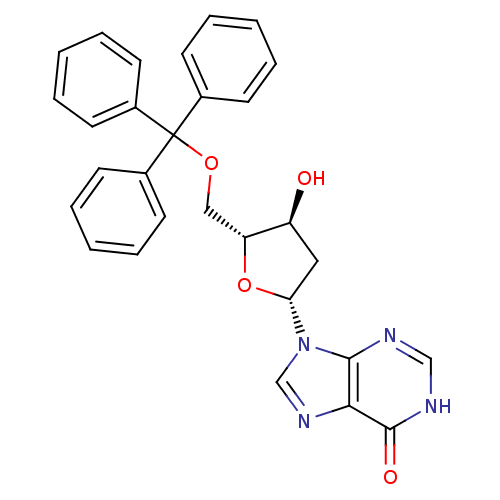
(5'-O-trityl-2'deoxyinosine | CHEMBL209655)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COC(c1ccccc1)(c1ccccc1)c1ccccc1)n1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C29H26N4O4/c34-23-16-25(33-19-32-26-27(33)30-18-31-28(26)35)37-24(23)17-36-29(20-10-4-1-5-11-20,21-12-6-2-7-13-21)22-14-8-3-9-15-22/h1-15,18-19,23-25,34H,16-17H2,(H,30,31,35)/t23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194171
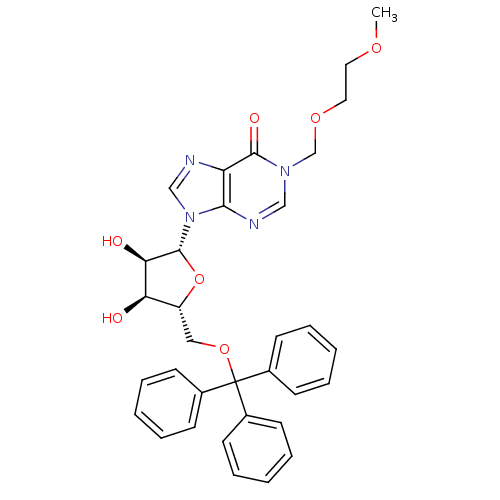
(1-[(2-methoxyethoxy)methyl]-5'-O-tritylinosine | C...)Show SMILES COCCOCn1cnc2n(cnc2c1=O)[C@@H]1O[C@H](COC(c2ccccc2)(c2ccccc2)c2ccccc2)[C@@H](O)[C@H]1O Show InChI InChI=1S/C33H34N4O7/c1-41-17-18-42-22-36-20-35-30-27(31(36)40)34-21-37(30)32-29(39)28(38)26(44-32)19-43-33(23-11-5-2-6-12-23,24-13-7-3-8-14-24)25-15-9-4-10-16-25/h2-16,20-21,26,28-29,32,38-39H,17-19,22H2,1H3/t26-,28-,29-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194169
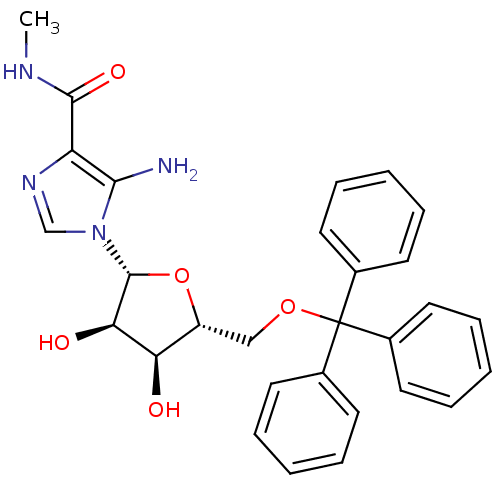
(5-amino-1-(5-O-trityl-beta-D-ribofuranosyl)imidazo...)Show SMILES CNC(=O)c1ncn([C@@H]2O[C@H](COC(c3ccccc3)(c3ccccc3)c3ccccc3)[C@@H](O)[C@H]2O)c1N Show InChI InChI=1S/C29H30N4O5/c1-31-27(36)23-26(30)33(18-32-23)28-25(35)24(34)22(38-28)17-37-29(19-11-5-2-6-12-19,20-13-7-3-8-14-20)21-15-9-4-10-16-21/h2-16,18,22,24-25,28,34-35H,17,30H2,1H3,(H,31,36)/t22-,24-,25-,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194183

(5'-O-trityl-2',3'-secoinosine | CHEMBL386743)Show SMILES OC[C@@H](COC(c1ccccc1)(c1ccccc1)c1ccccc1)O[C@H](CO)n1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C29H28N4O5/c34-16-24(38-25(17-35)33-20-32-26-27(33)30-19-31-28(26)36)18-37-29(21-10-4-1-5-11-21,22-12-6-2-7-13-22)23-14-8-3-9-15-23/h1-15,19-20,24-25,34-35H,16-18H2,(H,30,31,36)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50194182
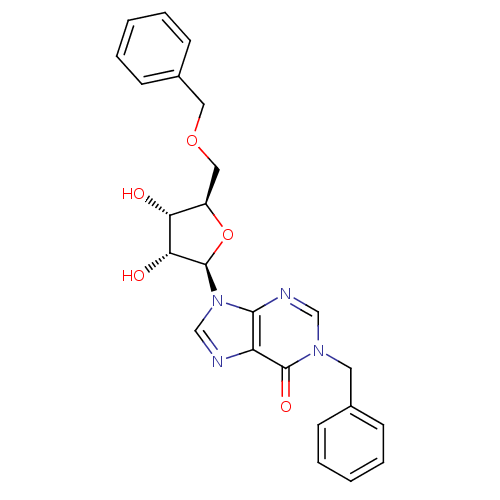
(1-benzyl-5'-O-benzylinosine | CHEMBL379953)Show SMILES O[C@@H]1[C@@H](COCc2ccccc2)O[C@H]([C@@H]1O)n1cnc2c1ncn(Cc1ccccc1)c2=O Show InChI InChI=1S/C24H24N4O5/c29-20-18(13-32-12-17-9-5-2-6-10-17)33-24(21(20)30)28-15-25-19-22(28)26-14-27(23(19)31)11-16-7-3-1-4-8-16/h1-10,14-15,18,20-21,24,29-30H,11-13H2/t18-,20-,21-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.)
Curated by ChEMBL
| Assay Description
Inhibition of human TPase in presence of 100 uM thymidine |
J Med Chem 49: 5562-70 (2006)
Article DOI: 10.1021/jm0605379
BindingDB Entry DOI: 10.7270/Q2WD4061 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data