 Found 689 hits with Last Name = 'heimrich' and Initial = 'e'
Found 689 hits with Last Name = 'heimrich' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50507816
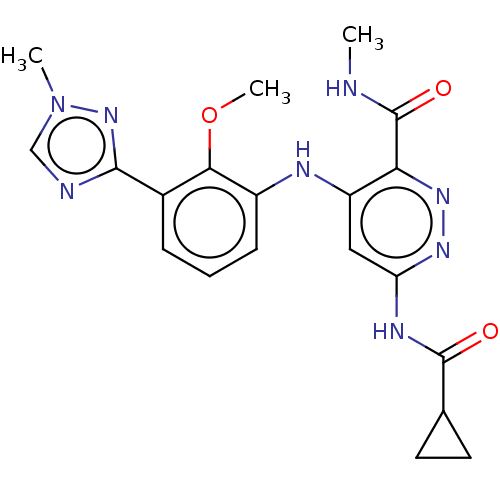
(Bms-986165 | Deucravacitinib)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1cccc(-c2ncn(C)n2)c1OC Show InChI InChI=1S/C20H22N8O3/c1-21-20(30)16-14(9-15(25-26-16)24-19(29)11-7-8-11)23-13-6-4-5-12(17(13)31-3)18-22-10-28(2)27-18/h4-6,9-11H,7-8H2,1-3H3,(H,21,30)(H2,23,24,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein labeled probe binding to His-tagged human TYK2 pseudokinase domain (575-869 residues) by Morrison titration based HTRF assa... |
J Med Chem 62: 8973-8995 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00444
BindingDB Entry DOI: 10.7270/Q2930XJS |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530394
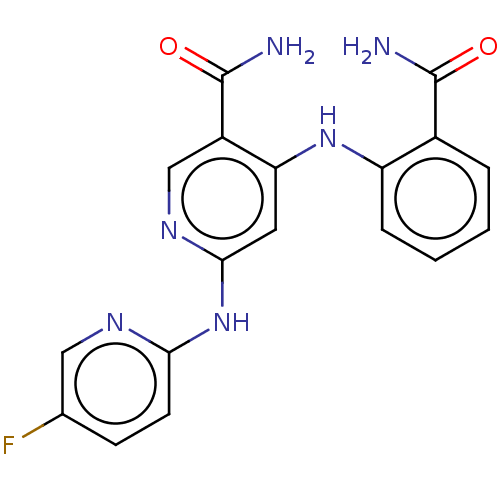
(CHEMBL4561663)Show SMILES NC(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H15FN6O2/c19-10-5-6-15(22-8-10)25-16-7-14(12(9-23-16)18(21)27)24-13-4-2-1-3-11(13)17(20)26/h1-9H,(H2,20,26)(H2,21,27)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530394

(CHEMBL4561663)Show SMILES NC(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H15FN6O2/c19-10-5-6-15(22-8-10)25-16-7-14(12(9-23-16)18(21)27)24-13-4-2-1-3-11(13)17(20)26/h1-9H,(H2,20,26)(H2,21,27)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50519523
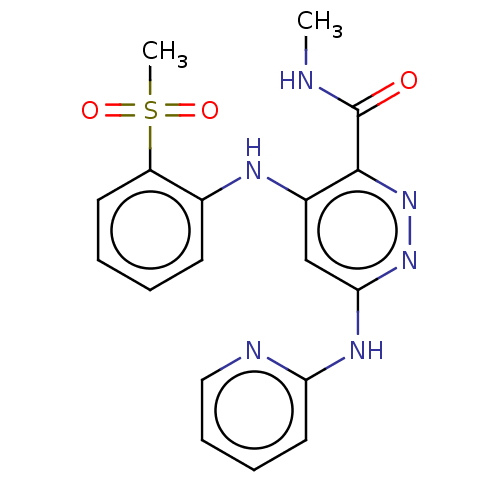
(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50519523

(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530395
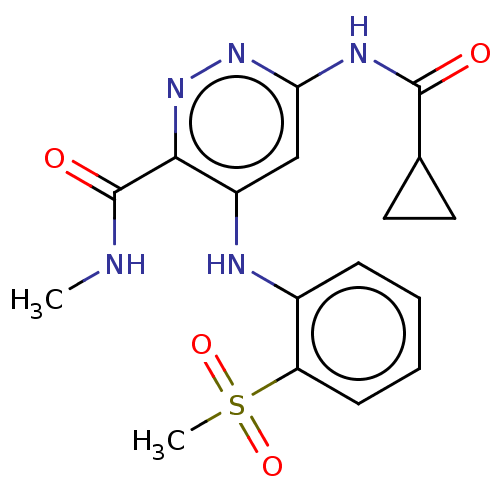
(CHEMBL4444178)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C17H19N5O4S/c1-18-17(24)15-12(9-14(21-22-15)20-16(23)10-7-8-10)19-11-5-3-4-6-13(11)27(2,25)26/h3-6,9-10H,7-8H2,1-2H3,(H,18,24)(H2,19,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530395

(CHEMBL4444178)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C17H19N5O4S/c1-18-17(24)15-12(9-14(21-22-15)20-16(23)10-7-8-10)19-11-5-3-4-6-13(11)27(2,25)26/h3-6,9-10H,7-8H2,1-2H3,(H,18,24)(H2,19,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysis |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50169441

(CHEMBL3806205)Show SMILES CCCCCC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C22H36NO4P/c1-2-3-4-5-6-17-7-8-19-14-20(10-9-18(19)13-17)21-11-12-22(23,15-21)16-27-28(24,25)26/h9-10,14,17,21H,2-8,11-13,15-16,23H2,1H3,(H2,24,25,26)/t17-,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM258470
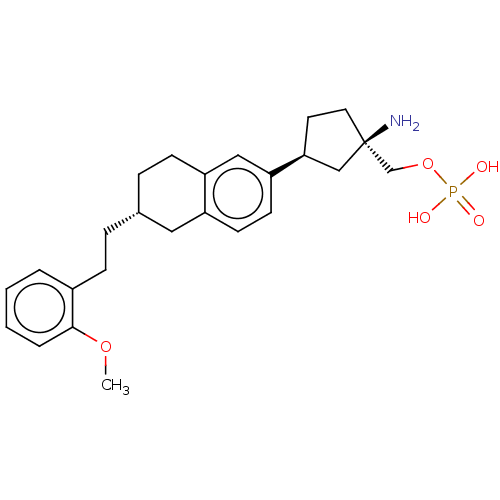
(US9522888, 697)Show SMILES COc1ccccc1CC[C@@H]1CCc2cc(ccc2C1)[C@H]1CC[C@](N)(COP(O)(O)=O)C1 |r| Show InChI InChI=1S/C25H34NO5P/c1-30-24-5-3-2-4-19(24)8-6-18-7-9-21-15-22(11-10-20(21)14-18)23-12-13-25(26,16-23)17-31-32(27,28)29/h2-5,10-11,15,18,23H,6-9,12-14,16-17,26H2,1H3,(H2,27,28,29)/t18-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM166831
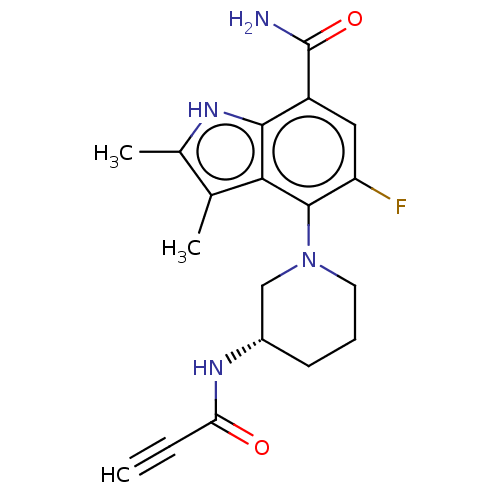
(US10604504, Example 242 | US11623921, Example 242 ...)Show SMILES Cc1[nH]c2c(cc(F)c(N3CCC[C@@H](C3)NC(=O)C#C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165319
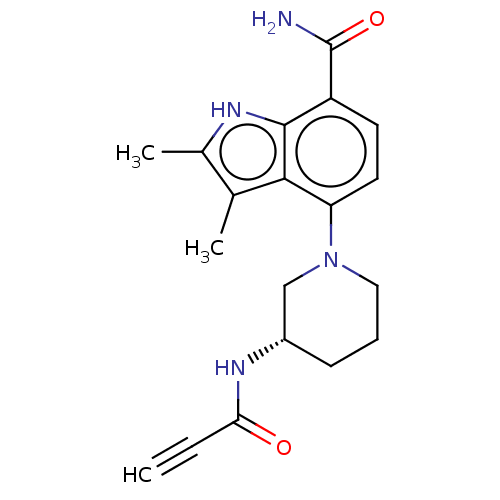
(US10604504, Example 115 | US11623921, Example 115 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCC[C@@H](C3)NC(=O)C#C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50507816

(Bms-986165 | Deucravacitinib)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1cccc(-c2ncn(C)n2)c1OC Show InChI InChI=1S/C20H22N8O3/c1-21-20(30)16-14(9-15(25-26-16)24-19(29)11-7-8-11)23-13-6-4-5-12(17(13)31-3)18-22-10-28(2)27-18/h4-6,9-11H,7-8H2,1-3H3,(H,21,30)(H2,23,24,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Allosteric inhibition of fluorescein labeled probe binding to His-tagged recombinant human TYK2 pseudokinase JH2 domain (575-869 residues) incubated ... |
J Med Chem 62: 8973-8995 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00444
BindingDB Entry DOI: 10.7270/Q2930XJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194718
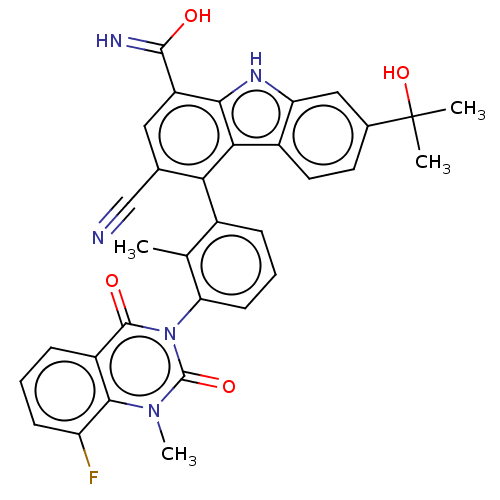
(CHEMBL3899411)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(cc(C(O)=N)c2[nH]c3cc(ccc3c12)C(C)(C)O)C#N |(-2.03,-1.34,;-1.6,.13,;-.1,.5,;.33,1.98,;-.74,3.09,;-2.23,2.73,;-2.66,1.25,;-4.16,.88,;-4.59,-.6,;-3.52,-1.71,;-6.08,-.97,;-6.51,-2.44,;-7.15,.15,;-8.65,-.22,;-9.08,-1.7,;-9.71,.89,;-9.28,2.37,;-7.78,2.74,;-6.72,1.63,;-5.22,1.99,;-4.79,3.47,;.96,-.61,;.53,-2.09,;1.6,-3.2,;3.09,-2.84,;4.16,-3.95,;5.66,-3.58,;3.73,-5.43,;3.52,-1.36,;5.06,-1.52,;6.34,-.67,;7.62,.19,;7.51,1.73,;6.13,2.41,;4.85,1.55,;4.95,.01,;2.46,-.24,;8.79,2.58,;7.94,3.86,;9.65,1.3,;10.07,3.44,;-.96,-2.46,;-2.46,-2.82,)| Show InChI InChI=1S/C33H26FN5O4/c1-16-19(7-6-10-25(16)39-31(41)21-8-5-9-23(34)29(21)38(4)32(39)42)26-17(15-35)13-22(30(36)40)28-27(26)20-12-11-18(33(2,3)43)14-24(20)37-28/h5-14,37,43H,1-4H3,(H2,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human PBMC assessed as reduction in TNFalpha expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human peripheral B cells assessed as reduction in CD86 surface expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530383
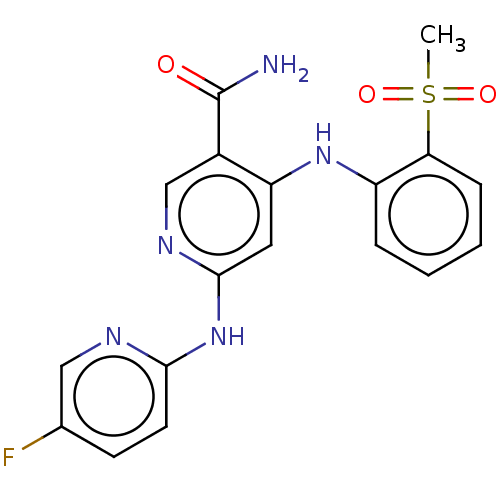
(CHEMBL4530719)Show SMILES CS(=O)(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H16FN5O3S/c1-28(26,27)15-5-3-2-4-13(15)23-14-8-17(22-10-12(14)18(20)25)24-16-7-6-11(19)9-21-16/h2-10H,1H3,(H2,20,25)(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530383

(CHEMBL4530719)Show SMILES CS(=O)(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H16FN5O3S/c1-28(26,27)15-5-3-2-4-13(15)23-14-8-17(22-10-12(14)18(20)25)24-16-7-6-11(19)9-21-16/h2-10H,1H3,(H2,20,25)(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194722
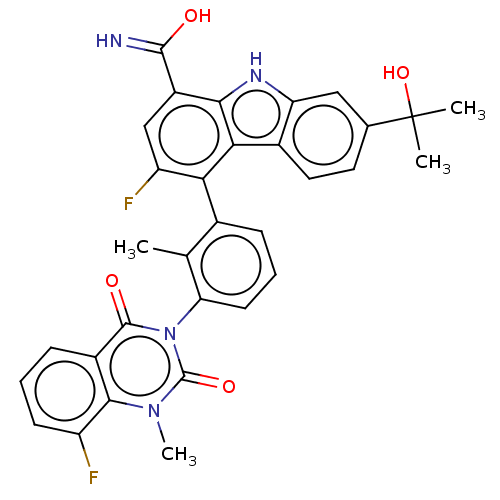
(CHEMBL3896019)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(F)cc(C(O)=N)c2[nH]c3cc(ccc3c12)C(C)(C)O |(-2.05,-1.45,;-1.63,.03,;-.14,.41,;.29,1.89,;-.78,2.99,;-2.28,2.62,;-2.7,1.14,;-4.2,.77,;-4.62,-.71,;-3.55,-1.82,;-6.11,-1.09,;-6.54,-2.57,;-7.18,.02,;-8.68,-.35,;-9.1,-1.84,;-9.75,.75,;-9.32,2.23,;-7.83,2.61,;-6.76,1.5,;-5.27,1.87,;-4.84,3.35,;.93,-.7,;.51,-2.18,;-.98,-2.55,;1.58,-3.29,;3.08,-2.91,;4.15,-4.02,;5.64,-3.65,;3.72,-5.5,;3.5,-1.43,;5.03,-1.59,;6.31,-.73,;7.58,.13,;7.47,1.67,;6.09,2.34,;4.81,1.48,;4.92,-.06,;2.43,-.33,;8.75,2.53,;7.89,3.81,;9.61,1.25,;10.03,3.39,)| Show InChI InChI=1S/C32H26F2N4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-21(33)28(19)37(4)31(38)41)25-22(34)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-14,36,42H,1-4H3,(H2,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530394

(CHEMBL4561663)Show SMILES NC(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H15FN6O2/c19-10-5-6-15(22-8-10)25-16-7-14(12(9-23-16)18(21)27)24-13-4-2-1-3-11(13)17(20)26/h1-9H,(H2,20,26)(H2,21,27)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530394

(CHEMBL4561663)Show SMILES NC(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H15FN6O2/c19-10-5-6-15(22-8-10)25-16-7-14(12(9-23-16)18(21)27)24-13-4-2-1-3-11(13)17(20)26/h1-9H,(H2,20,26)(H2,21,27)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530391
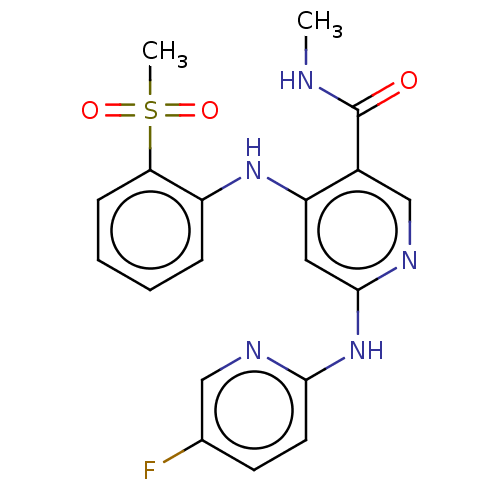
(CHEMBL4444169)Show SMILES [2H]C([2H])([2H])NC(=O)c1cnc(Nc2ccc(F)cn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C19H18FN5O3S/c1-21-19(26)13-11-23-18(25-17-8-7-12(20)10-22-17)9-15(13)24-14-5-3-4-6-16(14)29(2,27)28/h3-11H,1-2H3,(H,21,26)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530391

(CHEMBL4444169)Show SMILES [2H]C([2H])([2H])NC(=O)c1cnc(Nc2ccc(F)cn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C19H18FN5O3S/c1-21-19(26)13-11-23-18(25-17-8-7-12(20)10-22-17)9-15(13)24-14-5-3-4-6-16(14)29(2,27)28/h3-11H,1-2H3,(H,21,26)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194720
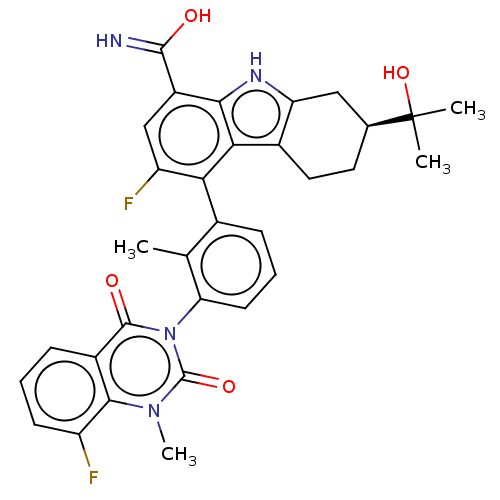
(CHEMBL3900554)Show SMILES [H][C@@]1(CCc2c(C1)[nH]c1c(cc(F)c(-c3cccc(c3C)-n3c(=O)n(C)c4c(F)cccc4c3=O)c21)C(O)=N)C(C)(C)O |r,wU:1.0,(7.64,3.3,;7.24,1.81,;5.84,2.47,;4.58,1.59,;4.71,.05,;6.1,-.6,;7.37,.28,;4.84,-1.48,;3.3,-1.34,;2.9,-2.83,;1.41,-3.22,;.33,-2.13,;-1.16,-2.53,;.73,-.64,;-.36,.45,;.05,1.93,;-1.04,3.03,;-2.53,2.63,;-2.93,1.15,;-1.85,.05,;-2.25,-1.43,;-4.42,.75,;-4.82,-.73,;-3.74,-1.83,;-6.31,-1.13,;-6.71,-2.62,;-7.4,-.04,;-8.89,-.43,;-9.29,-1.92,;-9.97,.66,;-9.57,2.15,;-8.08,2.54,;-6.99,1.45,;-5.51,1.84,;-5.1,3.33,;2.22,-.25,;3.99,-3.92,;5.48,-3.53,;3.58,-5.41,;8.77,1.67,;8.91,3.21,;8.63,.14,;10.3,1.53,)| Show InChI InChI=1S/C32H30F2N4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-21(33)28(19)37(4)31(38)41)25-22(34)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-10,14,16,36,42H,11-13H2,1-4H3,(H2,35,39)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194720

(CHEMBL3900554)Show SMILES [H][C@@]1(CCc2c(C1)[nH]c1c(cc(F)c(-c3cccc(c3C)-n3c(=O)n(C)c4c(F)cccc4c3=O)c21)C(O)=N)C(C)(C)O |r,wU:1.0,(7.64,3.3,;7.24,1.81,;5.84,2.47,;4.58,1.59,;4.71,.05,;6.1,-.6,;7.37,.28,;4.84,-1.48,;3.3,-1.34,;2.9,-2.83,;1.41,-3.22,;.33,-2.13,;-1.16,-2.53,;.73,-.64,;-.36,.45,;.05,1.93,;-1.04,3.03,;-2.53,2.63,;-2.93,1.15,;-1.85,.05,;-2.25,-1.43,;-4.42,.75,;-4.82,-.73,;-3.74,-1.83,;-6.31,-1.13,;-6.71,-2.62,;-7.4,-.04,;-8.89,-.43,;-9.29,-1.92,;-9.97,.66,;-9.57,2.15,;-8.08,2.54,;-6.99,1.45,;-5.51,1.84,;-5.1,3.33,;2.22,-.25,;3.99,-3.92,;5.48,-3.53,;3.58,-5.41,;8.77,1.67,;8.91,3.21,;8.63,.14,;10.3,1.53,)| Show InChI InChI=1S/C32H30F2N4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-21(33)28(19)37(4)31(38)41)25-22(34)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-10,14,16,36,42H,11-13H2,1-4H3,(H2,35,39)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165320
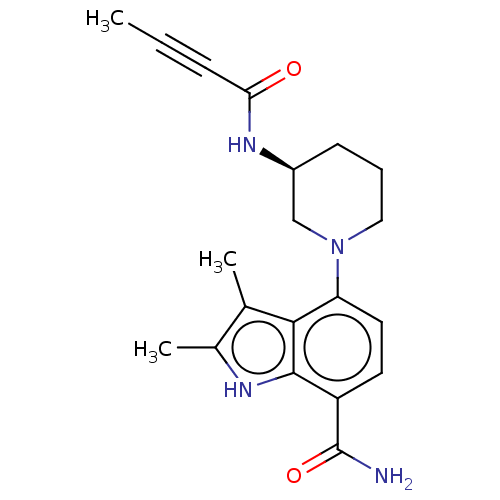
(US10604504, Example 116 | US11623921, Example 116 ...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1ccc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50519523

(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2/Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50519523

(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2/JAK1 in IFNalpha-stimulated human Kit225 cells by steady-glo luciferase assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50519523

(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2/Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50519523

(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2/JAK1 in IFNalpha-stimulated human Kit225 cells by steady-glo luciferase assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530403
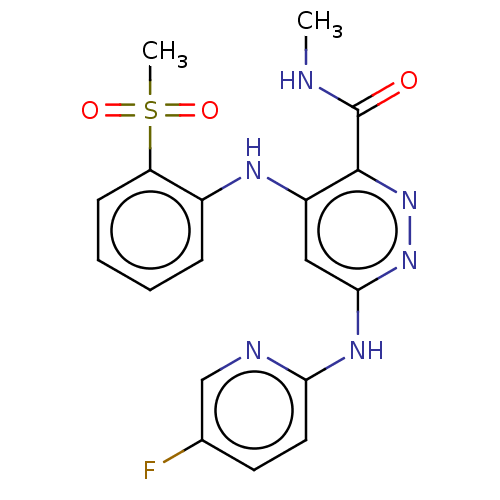
(CHEMBL4583185)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccc(F)cn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H17FN6O3S/c1-20-18(26)17-13(22-12-5-3-4-6-14(12)29(2,27)28)9-16(24-25-17)23-15-8-7-11(19)10-21-15/h3-10H,1-2H3,(H,20,26)(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530403

(CHEMBL4583185)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccc(F)cn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H17FN6O3S/c1-20-18(26)17-13(22-12-5-3-4-6-14(12)29(2,27)28)9-16(24-25-17)23-15-8-7-11(19)10-21-15/h3-10H,1-2H3,(H,20,26)(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165463
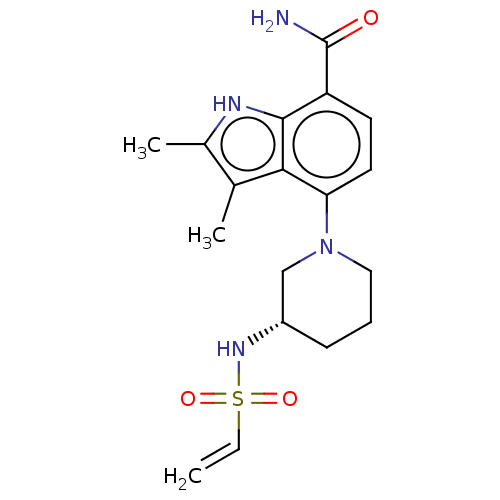
(US10604504, Example 141 | US11623921, Example 141 ...)Show SMILES Cc1[nH]c2c(ccc(N3CCC[C@@H](C3)NS(=O)(=O)C=C)c2c1C)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50194717
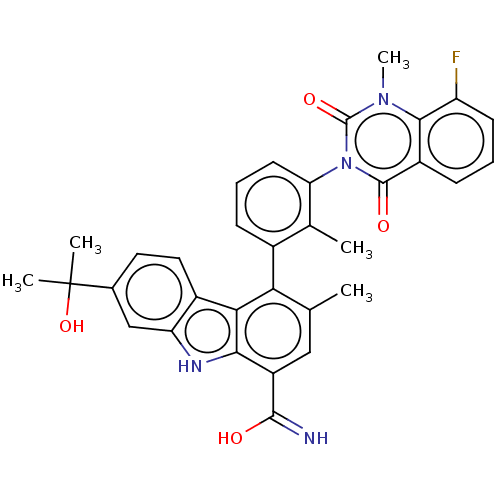
(CHEMBL3931086)Show SMILES Cc1cc(C(O)=N)c2[nH]c3cc(ccc3c2c1-c1cccc(c1C)-n1c(=O)n(C)c2c(F)cccc2c1=O)C(C)(C)O |(-.98,-2.55,;.51,-2.18,;1.58,-3.29,;3.08,-2.91,;4.15,-4.02,;5.64,-3.65,;3.72,-5.5,;3.5,-1.43,;5.03,-1.59,;6.31,-.73,;7.58,.13,;7.47,1.67,;6.09,2.34,;4.81,1.48,;4.92,-.06,;2.43,-.33,;.93,-.7,;-.14,.41,;.29,1.89,;-.78,2.99,;-2.28,2.62,;-2.7,1.14,;-1.63,.03,;-2.05,-1.45,;-4.2,.77,;-4.62,-.71,;-3.55,-1.82,;-6.11,-1.09,;-6.54,-2.57,;-7.18,.02,;-8.68,-.35,;-9.1,-1.84,;-9.75,.75,;-9.32,2.23,;-7.83,2.61,;-6.76,1.5,;-5.27,1.87,;-4.84,3.35,;8.75,2.53,;7.89,3.81,;9.61,1.25,;10.03,3.39,)| Show InChI InChI=1S/C33H29FN4O4/c1-16-14-22(30(35)39)28-27(20-13-12-18(33(3,4)42)15-24(20)36-28)26(16)19-8-7-11-25(17(19)2)38-31(40)21-9-6-10-23(34)29(21)37(5)32(38)41/h6-15,36,42H,1-5H3,(H2,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50463838
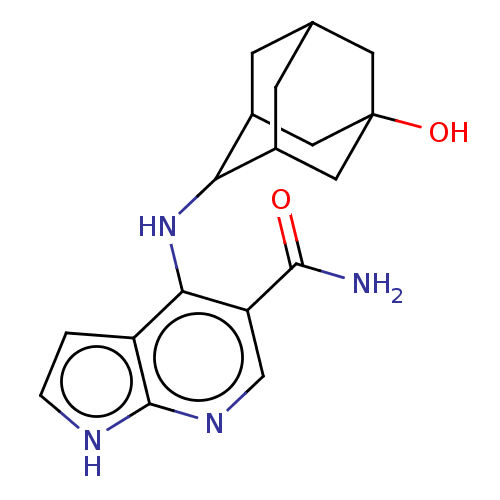
(CHEMBL4238926)Show SMILES NC(=O)c1cnc2[nH]ccc2c1NC1C2CC3CC1CC(O)(C3)C2 |TLB:13:14:22:17.18.19,12:13:22.16.17:19,THB:15:16:19:23.14.13,15:14:22.16.17:19,13:18:22:23.15.14,(62.43,-6.04,;61.1,-6.81,;61.1,-8.35,;59.76,-6.04,;59.75,-4.49,;58.42,-3.73,;57.08,-4.5,;55.61,-4.03,;54.71,-5.28,;55.62,-6.53,;57.09,-6.04,;58.42,-6.82,;58.42,-8.36,;57.14,-9.21,;57.12,-10.75,;56.11,-12.03,;54.69,-11.48,;54.69,-9.88,;55.72,-8.64,;54.38,-9.11,;54.39,-10.61,;53.04,-9.84,;53.19,-11.89,;55.71,-11.09,)| Show InChI InChI=1S/C18H22N4O2/c19-16(23)13-8-21-17-12(1-2-20-17)15(13)22-14-10-3-9-4-11(14)7-18(24,5-9)6-10/h1-2,8-11,14,24H,3-7H2,(H2,19,23)(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) in presence of Km ATP |
J Med Chem 62: 8973-8995 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00444
BindingDB Entry DOI: 10.7270/Q2930XJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM165318

(US10604504, Example 114 | US11623921, Example 114 ...)Show SMILES Cc1[nH]c2c(ccc(NC3CCN(CC3)C(=O)C#C)c2c1C)C(N)=O Show InChI InChI=1S/C19H22N4O2/c1-4-16(24)23-9-7-13(8-10-23)22-15-6-5-14(19(20)25)18-17(15)11(2)12(3)21-18/h1,5-6,13,21-22H,7-10H2,2-3H3,(H2,20,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human BTK using fluoresceinated peptide as substrate after 60 mins fluorescence assay |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of BTK in human memory B cells assessed as reduction in CD86 surface expression |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM164638

(BDBM166759 | US10604504, Example 223 | US11623921,...)Show SMILES CC#CC(=O)N[C@H]1CCCN(C1)c1c(F)cc(C(N)=O)c2[nH]c(C)c(C)c12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TEC (unknown origin) |
J Med Chem 62: 3228-3250 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00167
BindingDB Entry DOI: 10.7270/Q2ZK5M66 |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530398

(CHEMBL4526283)Show SMILES CNC(=O)c1cnc(Nc2ccc(F)cn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C19H18FN5O3S/c1-21-19(26)13-11-23-18(25-17-8-7-12(20)10-22-17)9-15(13)24-14-5-3-4-6-16(14)29(2,27)28/h3-11H,1-2H3,(H,21,26)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530398

(CHEMBL4526283)Show SMILES CNC(=O)c1cnc(Nc2ccc(F)cn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C19H18FN5O3S/c1-21-19(26)13-11-23-18(25-17-8-7-12(20)10-22-17)9-15(13)24-14-5-3-4-6-16(14)29(2,27)28/h3-11H,1-2H3,(H,21,26)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530396
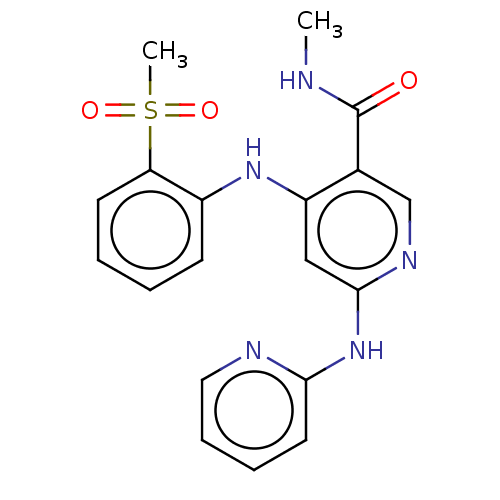
(CHEMBL4544320)Show SMILES CNC(=O)c1cnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C19H19N5O3S/c1-20-19(25)13-12-22-18(24-17-9-5-6-10-21-17)11-15(13)23-14-7-3-4-8-16(14)28(2,26)27/h3-12H,1-2H3,(H,20,25)(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530396

(CHEMBL4544320)Show SMILES CNC(=O)c1cnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C19H19N5O3S/c1-20-19(25)13-12-22-18(24-17-9-5-6-10-21-17)11-15(13)23-14-7-3-4-8-16(14)28(2,26)27/h3-12H,1-2H3,(H,20,25)(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein-labeled kinase tracer binding to His-TVMV-fused TYK2 JH2 domain (575 to 869 residues) (unknown origin) measured after 90 mi... |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50507816

(Bms-986165 | Deucravacitinib)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1cccc(-c2ncn(C)n2)c1OC Show InChI InChI=1S/C20H22N8O3/c1-21-20(30)16-14(9-15(25-26-16)24-19(29)11-7-8-11)23-13-6-4-5-12(17(13)31-3)18-22-10-28(2)27-18/h4-6,9-11H,7-8H2,1-3H3,(H,21,30)(H2,23,24,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein labeled probe binding to human recombinant JAK1 JH2 incubated for 1 hr by HTRF assay |
J Med Chem 62: 8973-8995 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00444
BindingDB Entry DOI: 10.7270/Q2930XJS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM230081
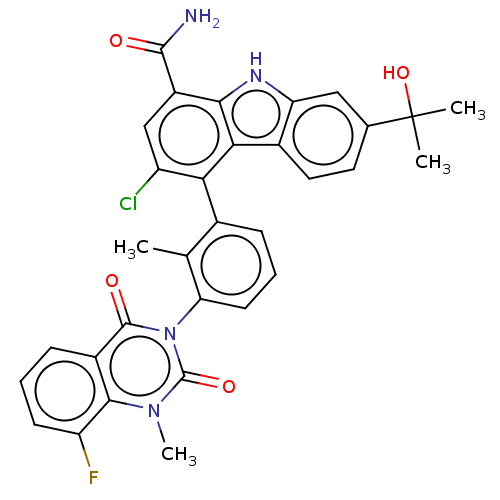
(US9334290, 1 | US9334290, 2)Show SMILES Cc1c(cccc1-n1c(=O)n(C)c2c(F)cccc2c1=O)-c1c(Cl)cc(C(N)=O)c2[nH]c3cc(ccc3c12)C(C)(C)O |(2.75,9.19,;1.42,8.42,;.08,9.19,;-1.25,8.42,;-1.25,6.88,;.08,6.11,;1.42,6.88,;2.75,6.11,;2.75,4.57,;1.42,3.8,;4.09,3.8,;4.09,2.26,;5.42,4.57,;6.75,3.8,;6.75,2.26,;8.09,4.57,;8.09,6.11,;6.75,6.88,;5.42,6.11,;4.09,6.88,;4.09,8.42,;.08,10.73,;1.42,11.5,;2.96,11.5,;1.42,13.04,;.08,13.81,;.08,15.35,;1.42,16.12,;-1.25,16.12,;-1.25,13.04,;-2.71,13.51,;-3.62,12.27,;-5.15,12.1,;-5.78,10.7,;-4.87,9.45,;-3.34,9.61,;-2.71,11.02,;-1.25,11.5,;-7.32,10.7,;-8.09,9.36,;-6.55,9.36,;-8.09,12.03,)| Show InChI InChI=1S/C32H26ClFN4O4/c1-15-17(7-6-10-24(15)38-30(40)19-8-5-9-22(34)28(19)37(4)31(38)41)25-21(33)14-20(29(35)39)27-26(25)18-12-11-16(32(2,3)42)13-23(18)36-27/h5-14,36,42H,1-4H3,(H2,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human His-tagged BTK expressed in baculovirus using fluoresceinated peptide incubated for 60 mins by fluorescen... |
J Med Chem 59: 9173-9200 (2016)
Article DOI: 10.1021/acs.jmedchem.6b01088
BindingDB Entry DOI: 10.7270/Q23T9K5H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data