

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting | Bioorg Med Chem 19: 1349-60 (2011) Article DOI: 10.1016/j.bmc.2010.11.051 BindingDB Entry DOI: 10.7270/Q2TT4TS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50520922 (CHEMBL4547780) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM31046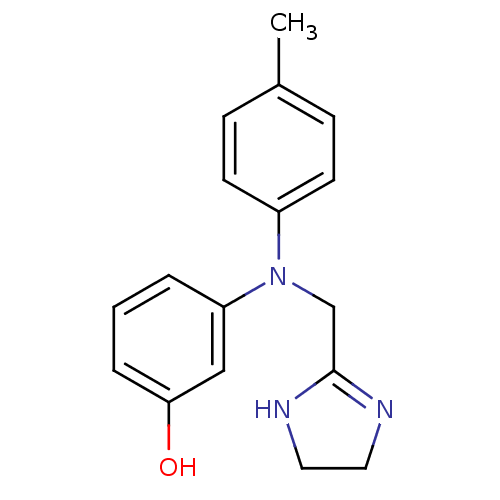 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50417067 (CHEMBL1258345) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting | Bioorg Med Chem 19: 1349-60 (2011) Article DOI: 10.1016/j.bmc.2010.11.051 BindingDB Entry DOI: 10.7270/Q2TT4TS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485347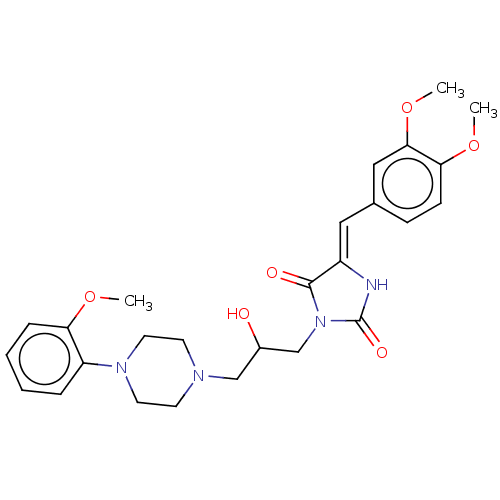 (CHEMBL2046970) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485355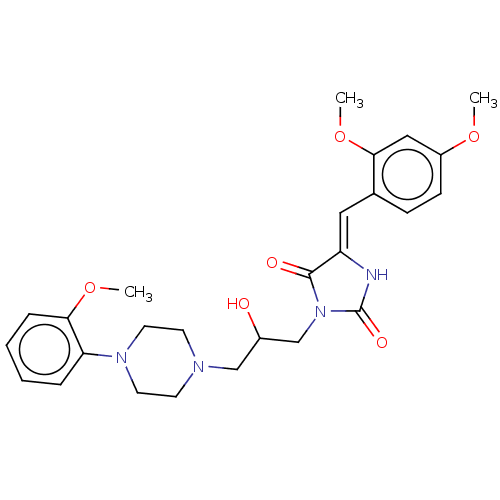 (CHEMBL2046969) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485360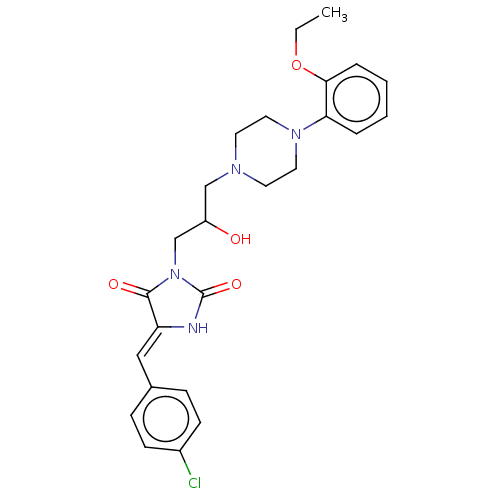 (CHEMBL2046968) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50417068 (CHEMBL1258457) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting | Bioorg Med Chem 19: 1349-60 (2011) Article DOI: 10.1016/j.bmc.2010.11.051 BindingDB Entry DOI: 10.7270/Q2TT4TS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485348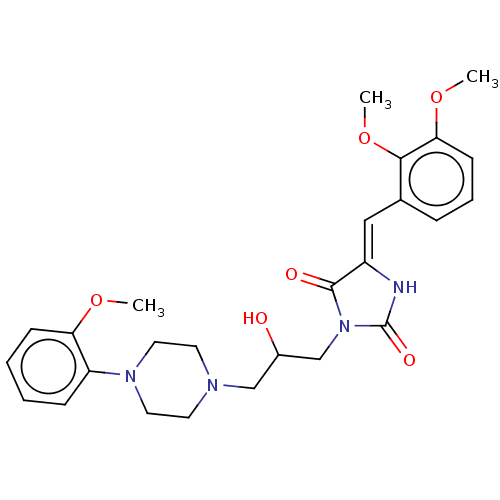 (CHEMBL2046967) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485349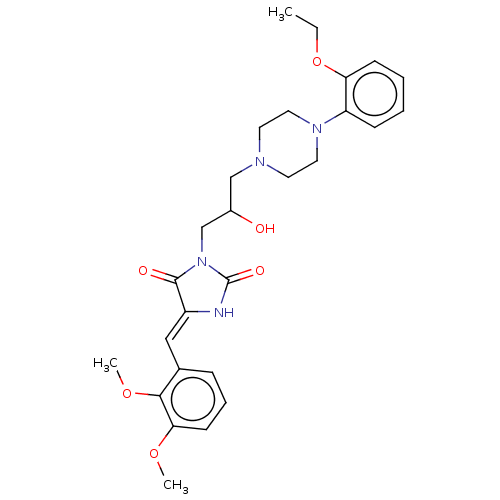 (CHEMBL2046966) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485356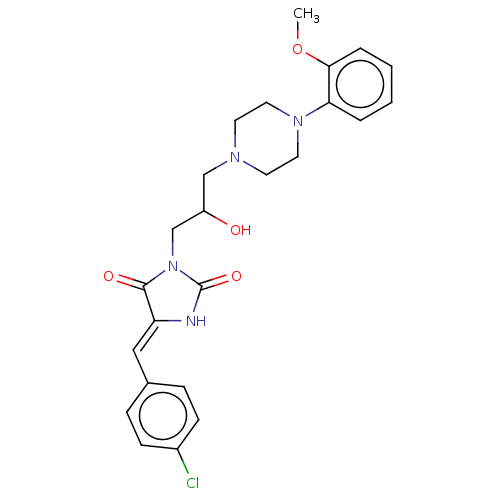 (CHEMBL2046965) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485350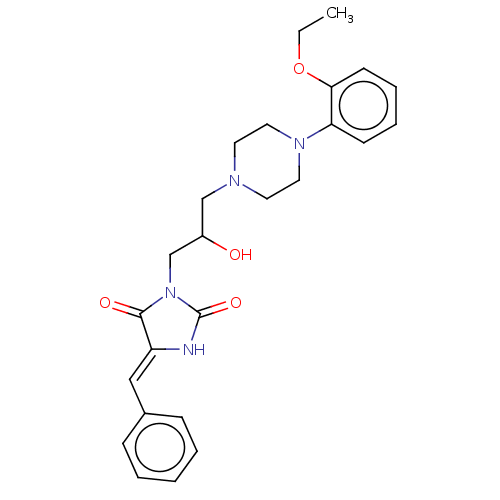 (CHEMBL2046964) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485351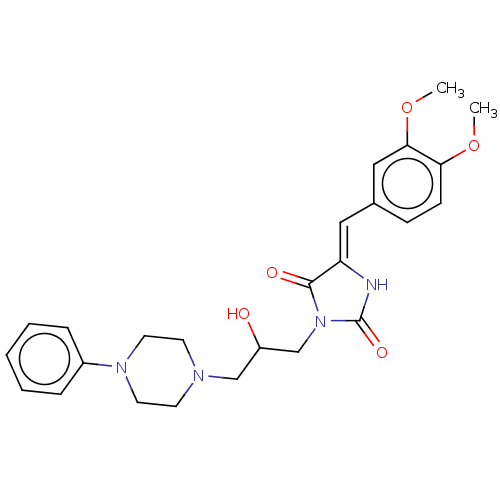 (CHEMBL2046963) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485357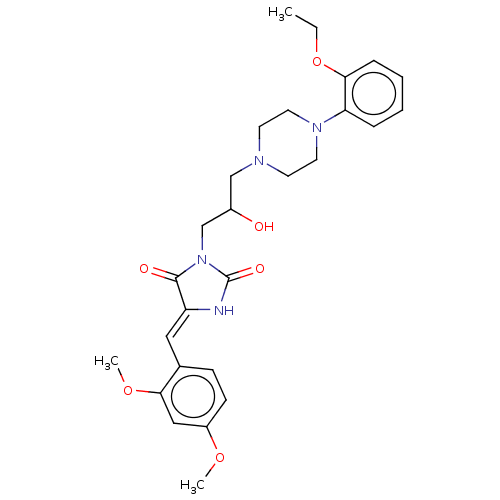 (CHEMBL2046962) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485359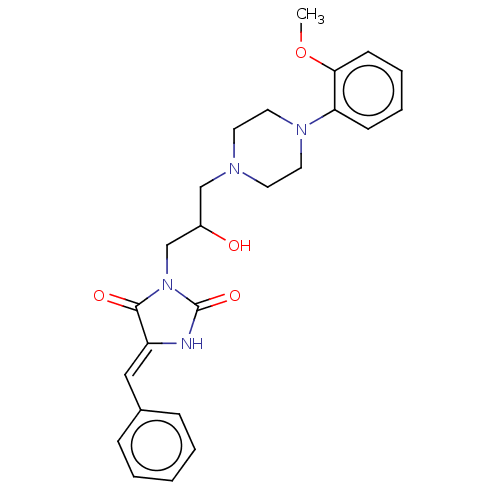 (CHEMBL2046961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50520924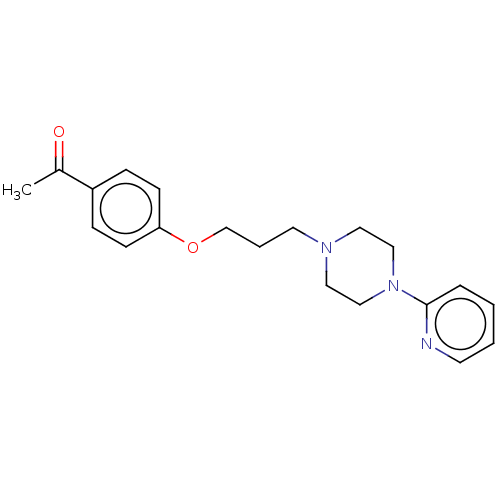 (CHEMBL4581913) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 315 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485354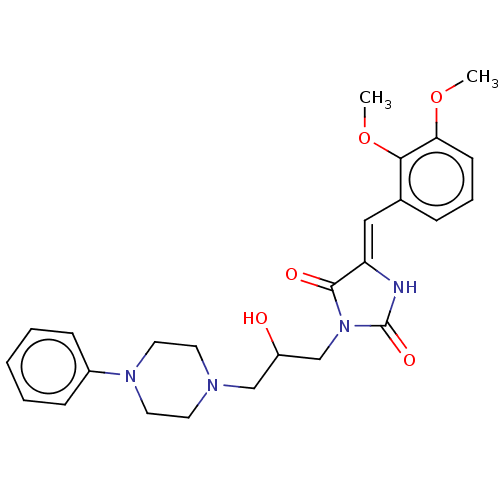 (CHEMBL2046960) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 319 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50405909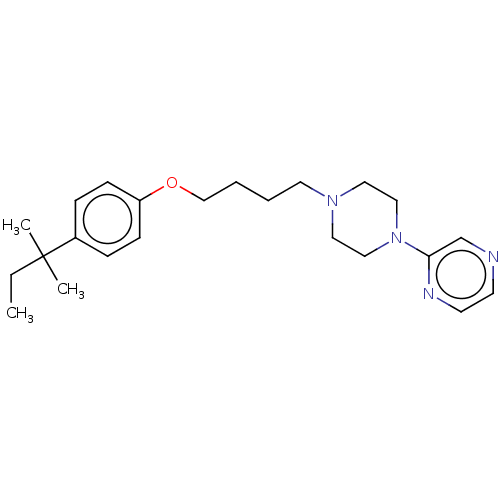 (CHEMBL4170784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50417066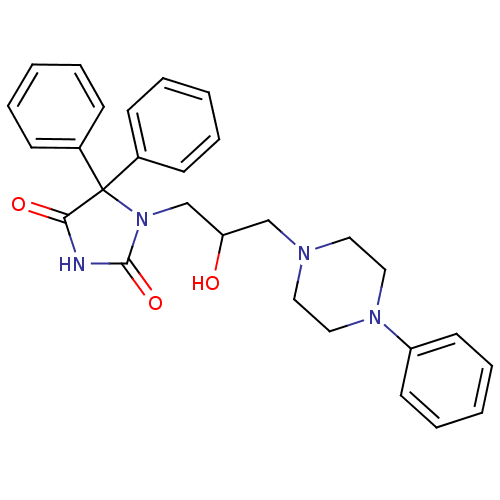 (CHEMBL1258344) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 732 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting | Bioorg Med Chem 19: 1349-60 (2011) Article DOI: 10.1016/j.bmc.2010.11.051 BindingDB Entry DOI: 10.7270/Q2TT4TS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485352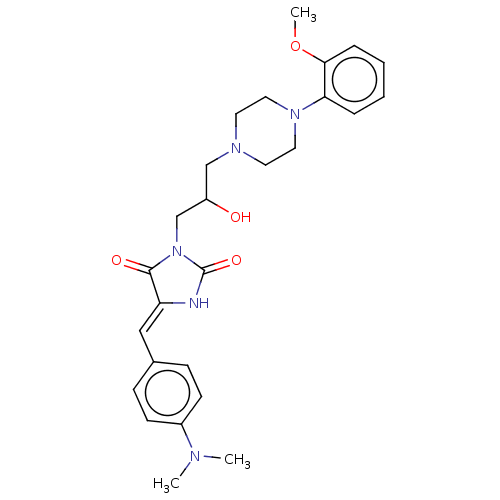 (CHEMBL2046959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 835 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50405708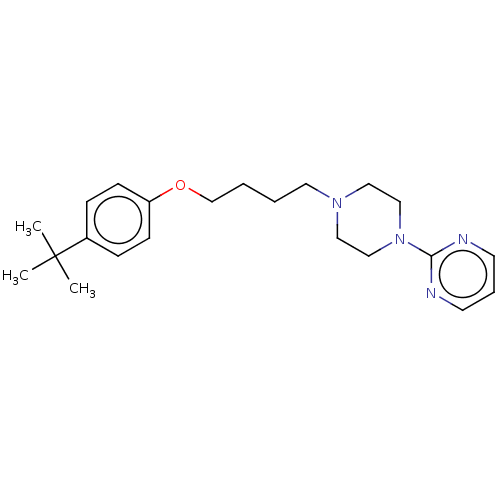 (CHEMBL4166945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 883 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50520935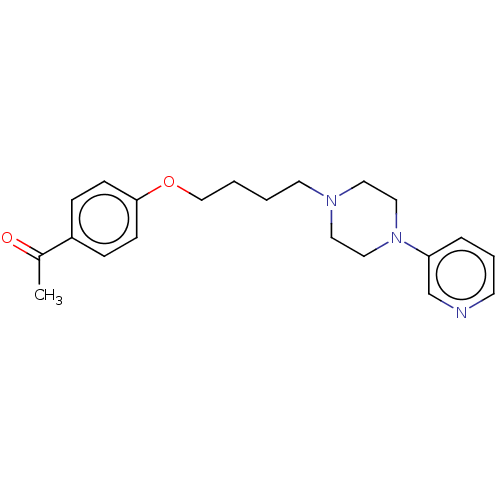 (CHEMBL4473431) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 908 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50520933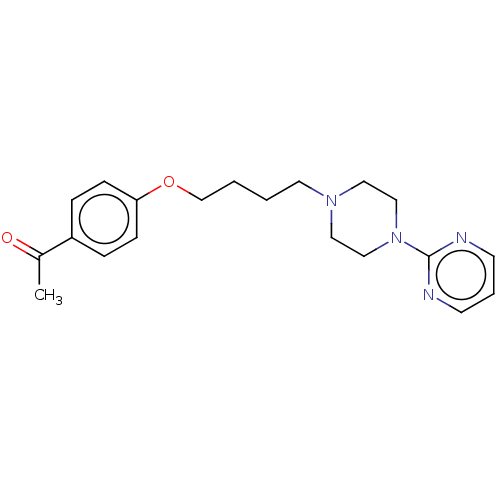 (CHEMBL4467220) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50456718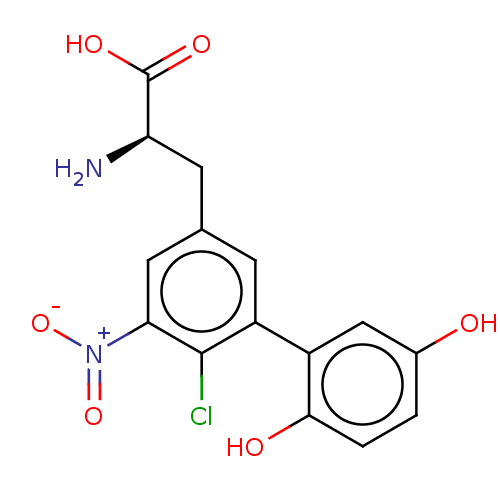 (CHEMBL4208162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA2(R) receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50520937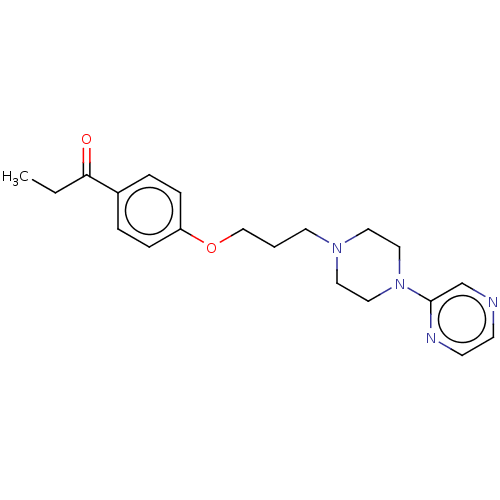 (CHEMBL4445569) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50456718 (CHEMBL4208162) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA2(Q) receptor flop isoform by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50405908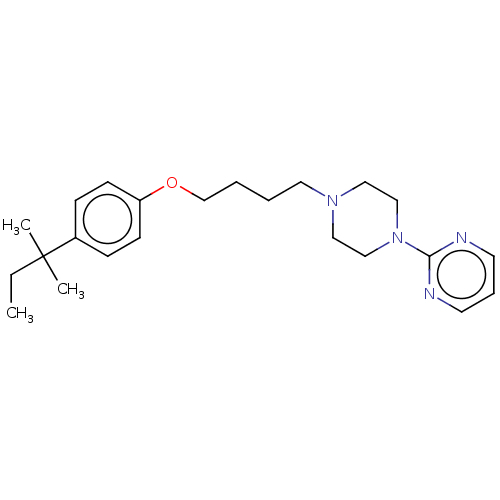 (CHEMBL4162840) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50520941 (CHEMBL4519189) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50405707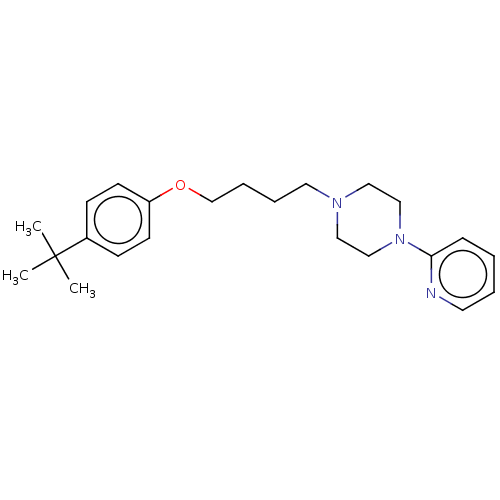 (CHEMBL4163913) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50520934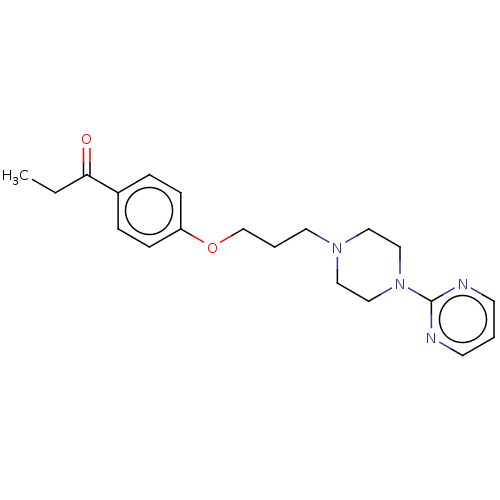 (CHEMBL4453108) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50405734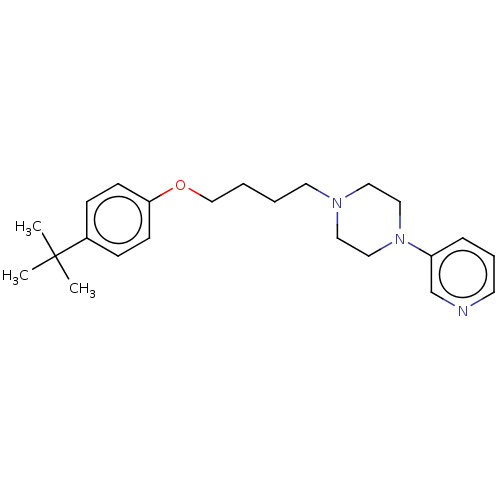 (CHEMBL4170350) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50520940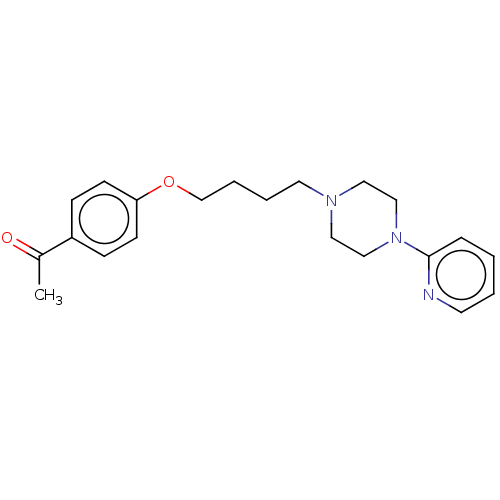 (CHEMBL4452580) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485358 (CHEMBL2046958) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50405712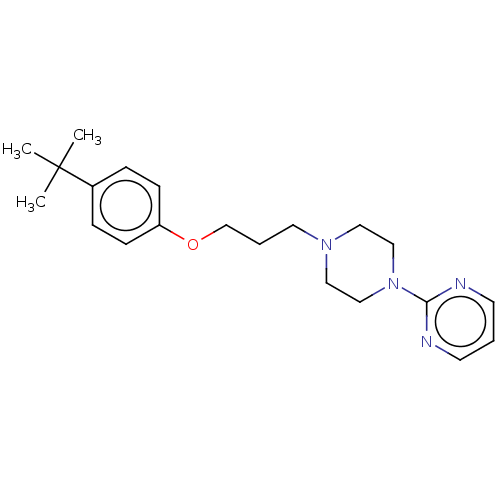 (CHEMBL4166262) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50483374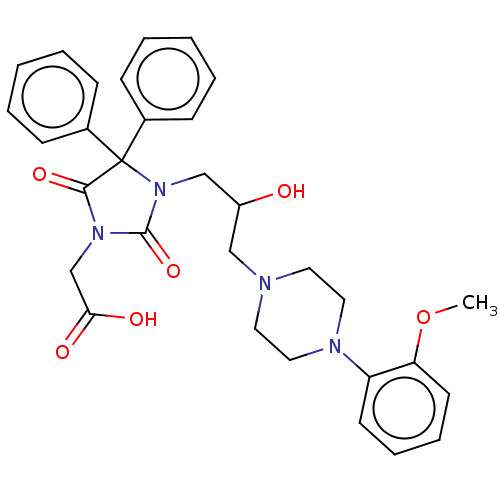 (CHEMBL1669478) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting | Bioorg Med Chem 19: 1349-60 (2011) Article DOI: 10.1016/j.bmc.2010.11.051 BindingDB Entry DOI: 10.7270/Q2TT4TS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50405702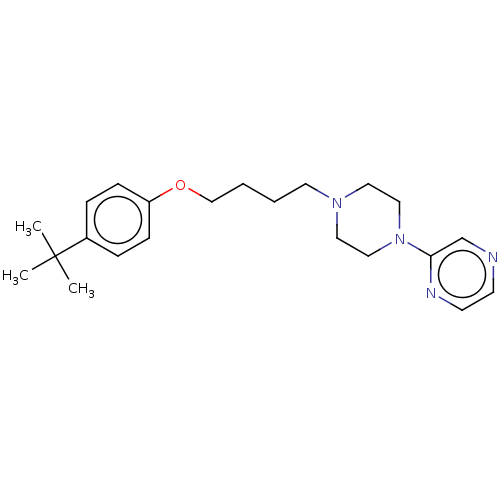 (CHEMBL4159046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50456718 (CHEMBL4208162) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA3 receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50485353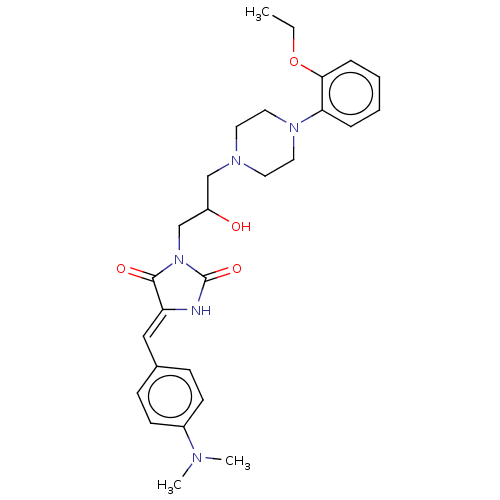 (CHEMBL2046971) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by beta scintillation counting | Bioorg Med Chem 20: 4245-57 (2012) Article DOI: 10.1016/j.bmc.2012.05.064 BindingDB Entry DOI: 10.7270/Q2862K90 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50405703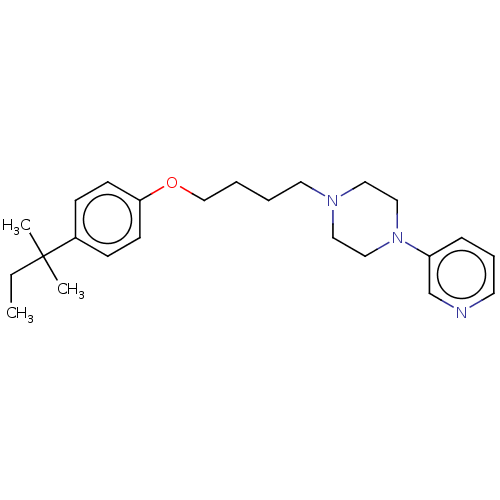 (CHEMBL4171151) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to at human H3 receptor | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127522 BindingDB Entry DOI: 10.7270/Q29K4FVC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50456716 (CHEMBL4207628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA2(R) receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50456717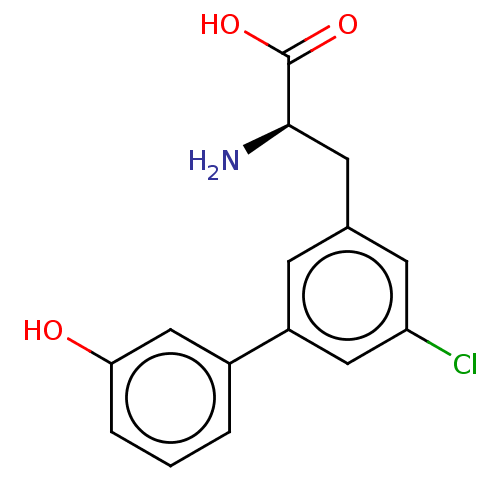 (CHEMBL4212533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA2(R) receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50456719 (CHEMBL4216018) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA2(R) receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50456720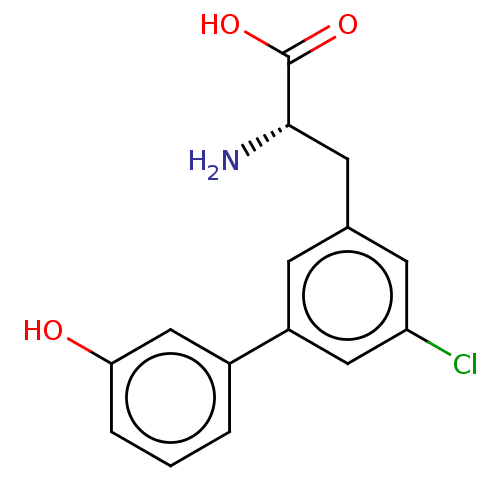 (CHEMBL4211059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA2(R) receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50456716 (CHEMBL4207628) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA3 receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50456719 (CHEMBL4216018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA3 receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 2 (Rattus norvegicus) | BDBM50456721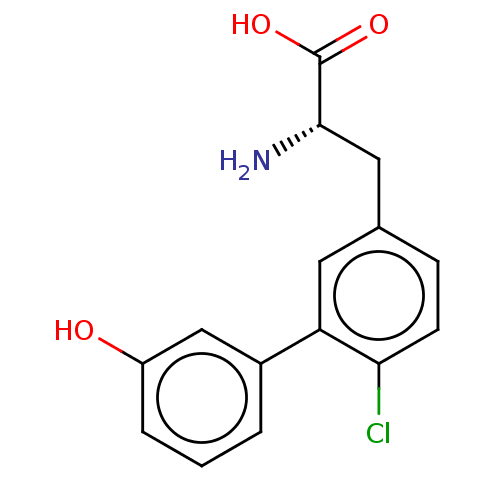 (CHEMBL4215471) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA2(R) receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50456720 (CHEMBL4211059) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA3 receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50456721 (CHEMBL4215471) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA3 receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor 3 (RAT) | BDBM50456717 (CHEMBL4212533) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]AMPA from rat GluA3 receptor flip isoform expressed in sf9 insect cell membranes by TopCount method | Eur J Med Chem 138: 874-883 (2017) Article DOI: 10.1016/j.ejmech.2017.07.007 BindingDB Entry DOI: 10.7270/Q2862K1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||