

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526301 (CHEMBL4456099) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526302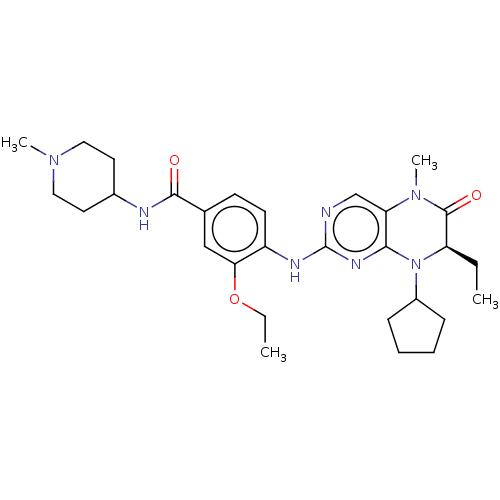 (CHEMBL4464826) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526296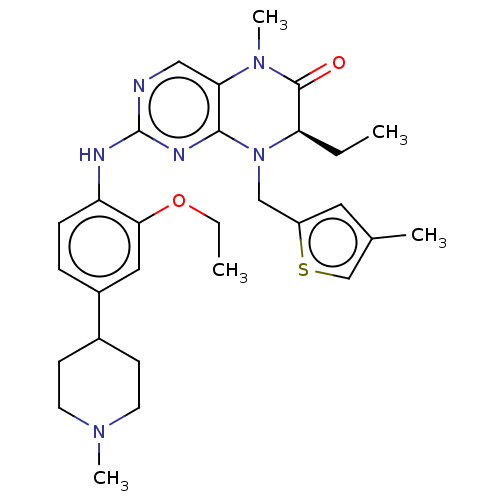 (CHEMBL4449858) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526309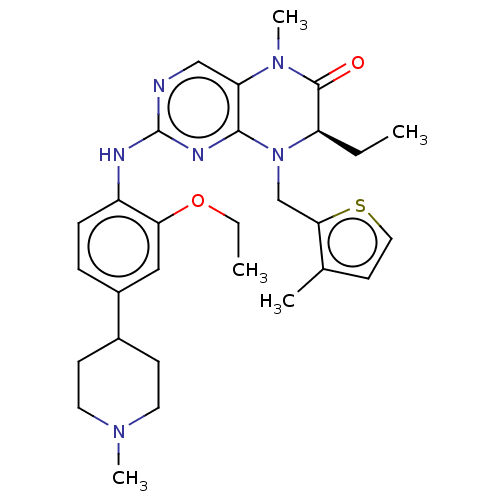 (CHEMBL4543331) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526309 (CHEMBL4543331) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50436850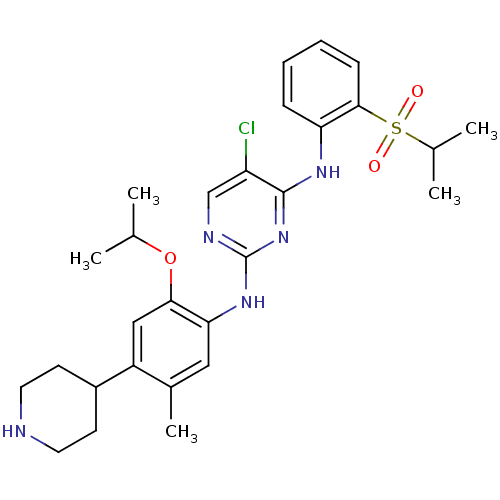 (CERITINIB | CHEMBL2403108 | LDK378 | US10053458, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of ALK F1174L mutant auto-phosphorylation in human Kelly cells after 3 hrs by MSD assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526303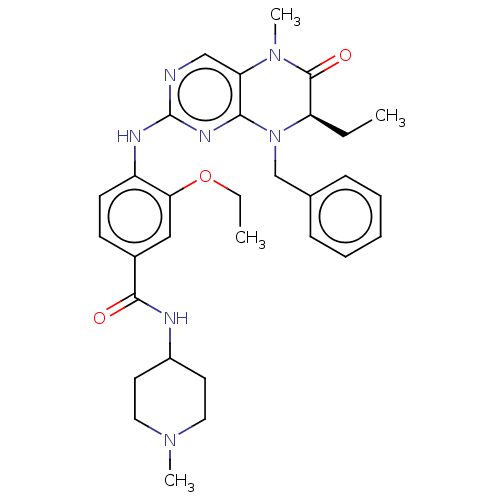 (CHEMBL4457809) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526295 (CHEMBL4469087) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526300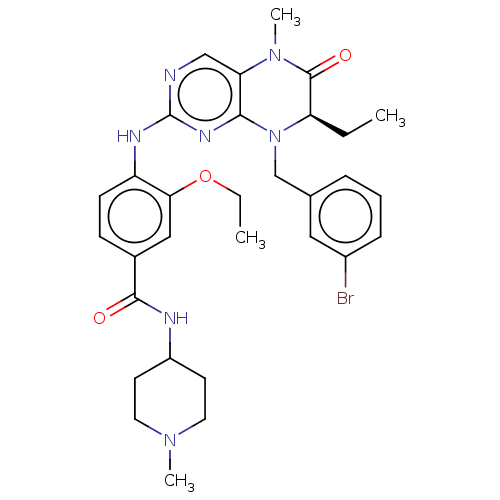 (CHEMBL4469756) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526296 (CHEMBL4449858) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526305 (CHEMBL4557730) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526297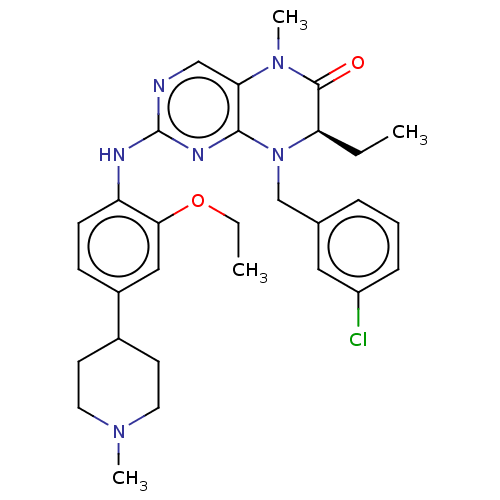 (CHEMBL4448214) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526307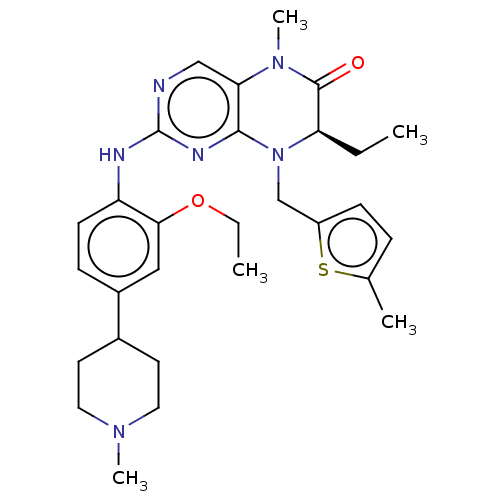 (CHEMBL4519103) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526296 (CHEMBL4449858) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal NanoLuc-tagged BRD4 BD1 (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engageme... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526295 (CHEMBL4469087) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526304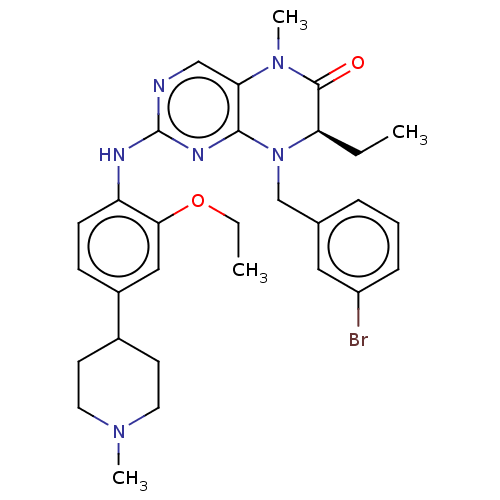 (CHEMBL4451065) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526301 (CHEMBL4456099) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal NanoLuc-tagged BRD4 BD1 (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engageme... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526309 (CHEMBL4543331) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal NanoLuc-tagged BRD4 BD1 (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engageme... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526299 (CHEMBL4454885) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526294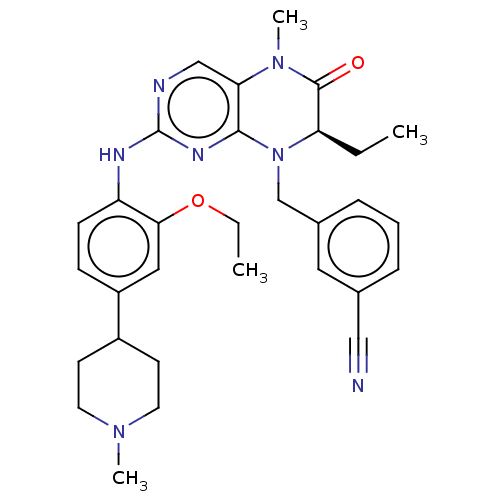 (CHEMBL4588843) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526308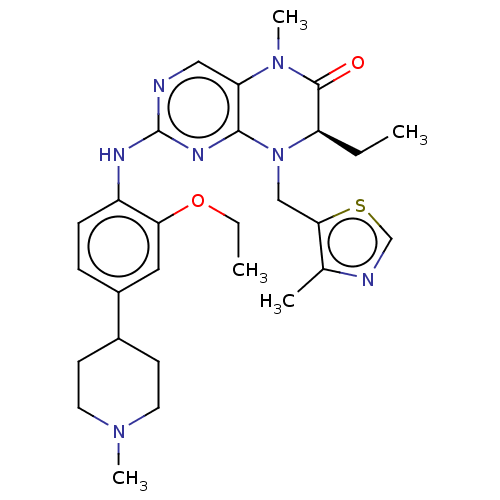 (CHEMBL4549308) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526302 (CHEMBL4464826) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of ALK (unknown origin) | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526303 (CHEMBL4457809) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526296 (CHEMBL4449858) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal NanoLuc-tagged ALK (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engagement as... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526300 (CHEMBL4469756) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526298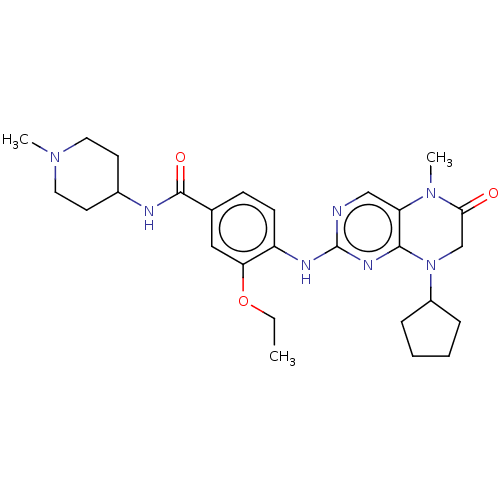 (CHEMBL4436006) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526304 (CHEMBL4451065) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526297 (CHEMBL4448214) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526295 (CHEMBL4469087) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal NanoLuc-tagged BRD4 BD1 (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engageme... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526306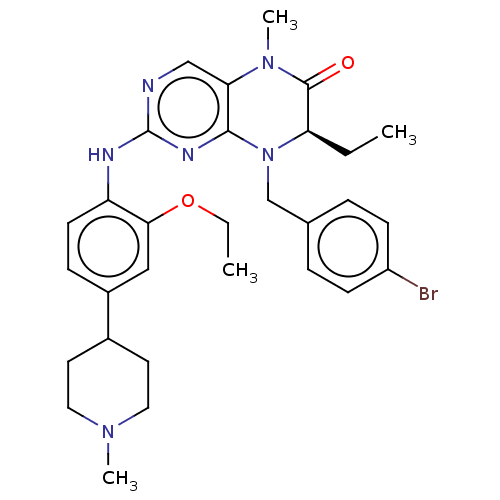 (CHEMBL4459025) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal NanoLuc-tagged ALK (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engagement as... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526309 (CHEMBL4543331) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal NanoLuc-tagged ALK (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engagement as... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526296 (CHEMBL4449858) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of ALK F1174L mutant auto-phosphorylation in human Kelly cells after 3 hrs by MSD assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526295 (CHEMBL4469087) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of full-length C-terminal NanoLuc-tagged ALK (unknown origin) expressed in human HEK293 cells after 2 hrs by NanoBRET target engagement as... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50112342 (CHEMBL3609312) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM50526294 (CHEMBL4588843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of PLK1 (unknown origin) using Ser/Thr 16 as substrate after 60 mins by Z-LYTE activity assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526310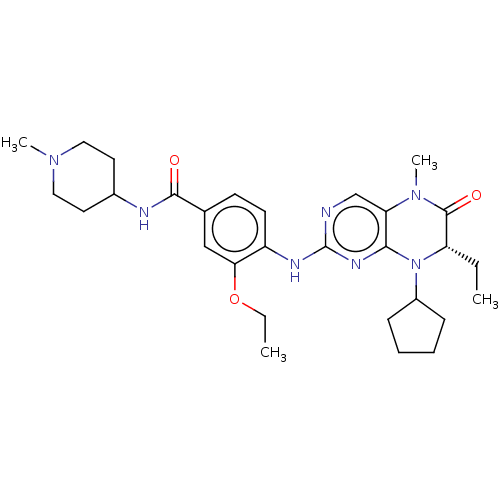 (CHEMBL4551421) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Displacement of kinase tracer 236 from human N-terminal GST-tagged ALK F1174L mutant (1058 to 1620 residues) after 60 mins by LanthaScreen Eu--kinase... | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of ALK F1174L mutant auto-phosphorylation in human Kelly cells after 3 hrs by MSD assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526296 (CHEMBL4449858) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to BRD4 (unknown origin) by isothermal calorimetry | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526302 (CHEMBL4464826) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to BRD4 (unknown origin) by isothermal calorimetry | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526301 (CHEMBL4456099) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to BRD4 (unknown origin) by isothermal calorimetry | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526300 (CHEMBL4469756) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to BRD4 (unknown origin) by isothermal calorimetry | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM25121 (4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to BRD4 (unknown origin) by isothermal calorimetry | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526294 (CHEMBL4588843) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to BRD4 (unknown origin) by isothermal calorimetry | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50526296 (CHEMBL4449858) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to human ALK F1174L mutant (1088 to 1409 residues) expressed in mammalian expression system by kinome scan-based assay | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50526295 (CHEMBL4469087) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Binding affinity to BRD4 (unknown origin) by isothermal calorimetry | J Med Chem 62: 2618-2637 (2019) Article DOI: 10.1021/acs.jmedchem.8b01947 BindingDB Entry DOI: 10.7270/Q28S4TBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |