

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuraminidase (Influenza A virus) | BDBM50482873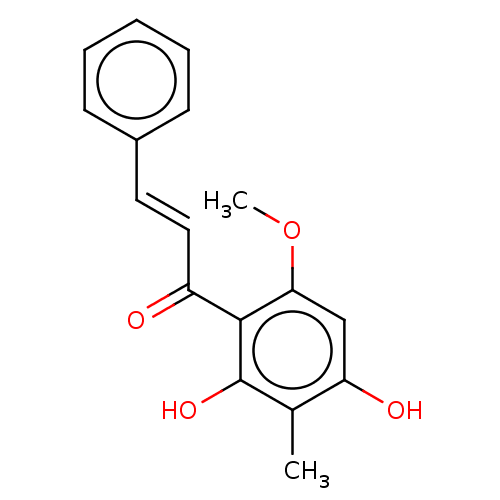 (CHEBI:70659 | CHEMBL1271362) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482872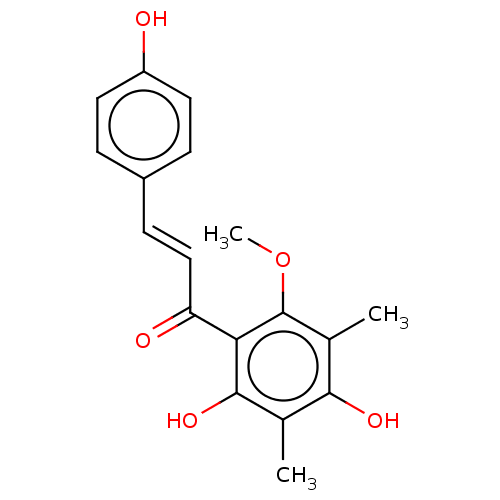 (CHEBI:70655 | CHEMBL1271157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482871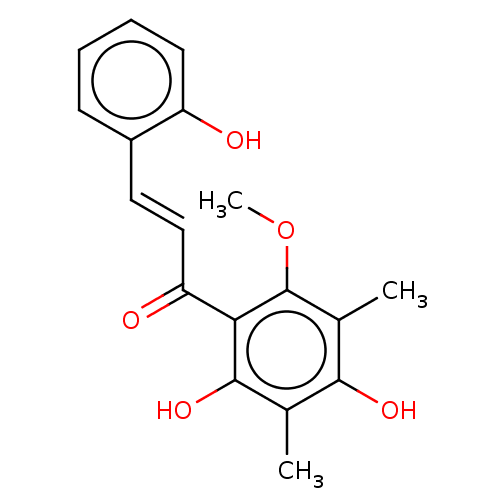 (CHEBI:66265 | CHEMBL509947) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482882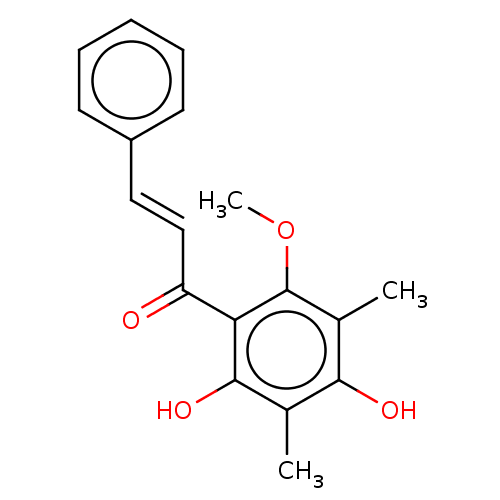 (CHEBI:70658 | CHEMBL463095) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) neuraminidase expressed in human 293T cells by Dixon plot analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323211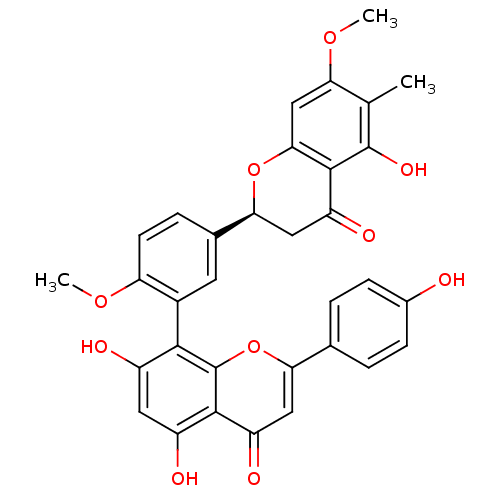 (2,3-dihydro-6-methylginkgetin | CHEMBL1208905) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323210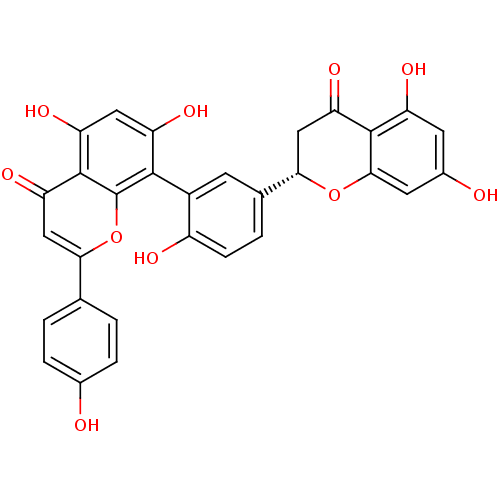 (2,3-dihydroamentoflavone | CHEMBL220741) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323198 (4'''-methylamentoflavone | CHEMBL220745 | podocarp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323195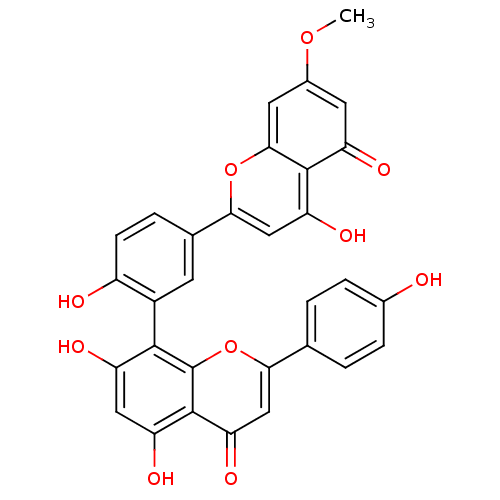 (CHEMBL255493 | sequoiaflavone) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50129952 (2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323197 (7''-O-methylamentoflavone | CHEMBL450522 | sotetsu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323196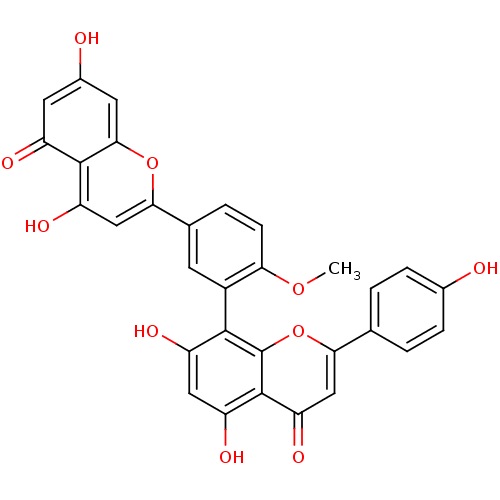 (4'-methylamentoflavone | CHEMBL378188 | bilobetin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482871 (CHEBI:66265 | CHEMBL509947) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323203 (CHEMBL1208903 | isoginkgetin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482872 (CHEBI:70655 | CHEMBL1271157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323199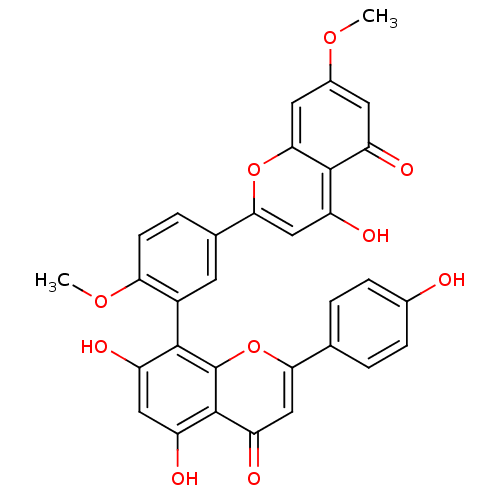 (5,7-dihydroxy-8-(5-(5-hydroxy-7-methoxy-4-oxo-4H-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323201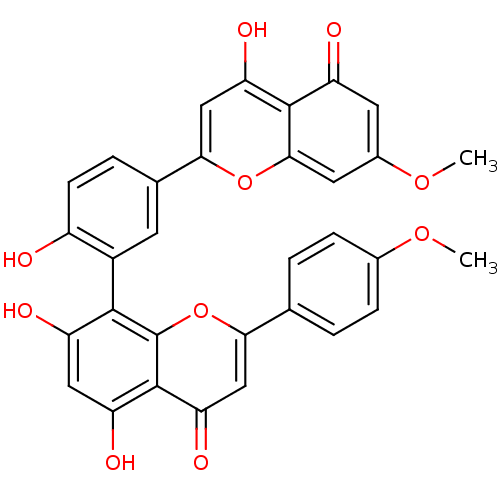 (CHEMBL144630 | podocarpusflavone B | putraflavone) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50323196 (4'-methylamentoflavone | CHEMBL378188 | bilobetin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50129952 (2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50323198 (4'''-methylamentoflavone | CHEMBL220745 | podocarp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50323195 (CHEMBL255493 | sequoiaflavone) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482882 (CHEBI:70658 | CHEMBL463095) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323200 (CHEMBL1208793 | amentoflavone-7,7''-dimethyl ether) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482872 (CHEBI:70655 | CHEMBL1271157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) wild type neuraminidase expressed in human 293T cells after 30 mins by spectrofluorimetr... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50323214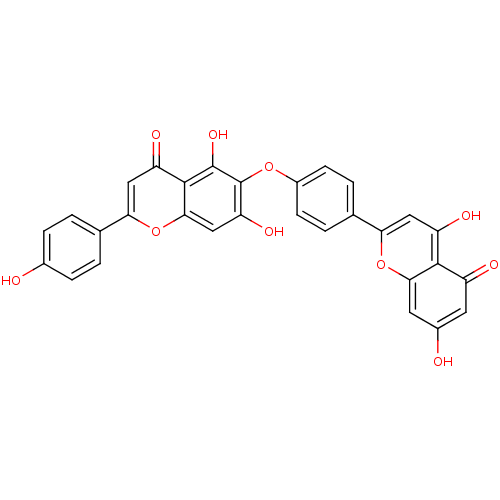 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482871 (CHEBI:66265 | CHEMBL509947) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/08/2009(H1N1)) wild type neuraminidase expressed in human 293T cells after 30 mins by spectrofluorimetr... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323213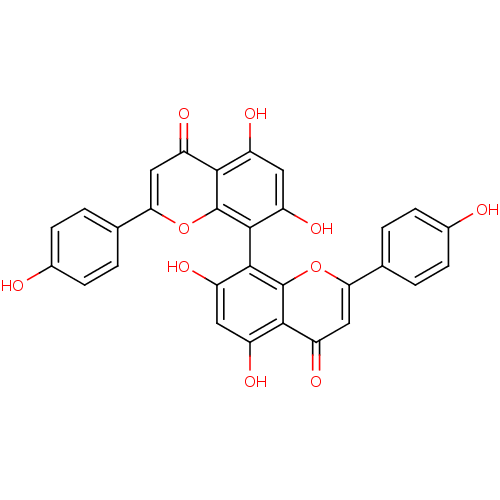 (CHEMBL1208973 | cupressuflavone) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323208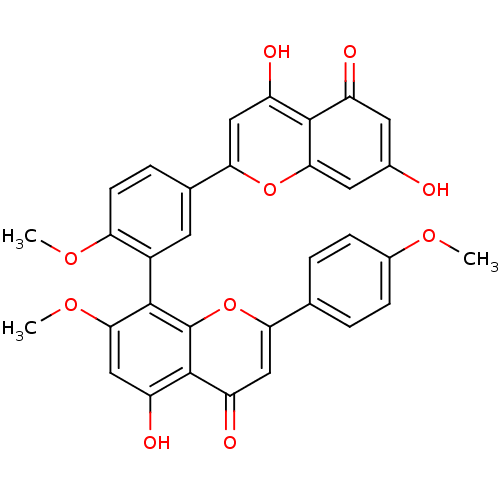 (CHEMBL453479 | kayaflavone) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323206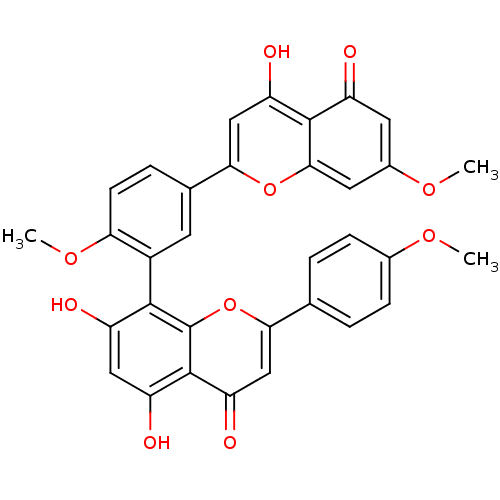 (CHEMBL208908 | sciadopitisin | sciadopitysin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323209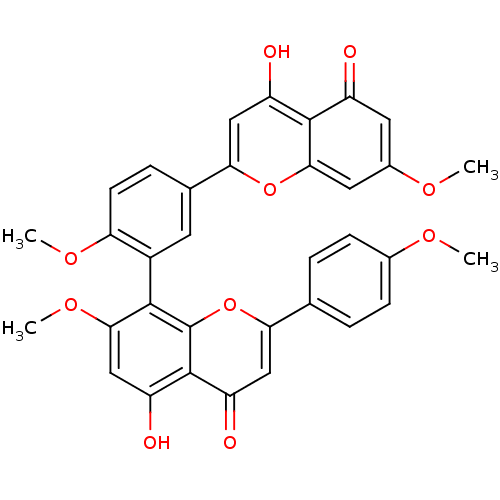 (7,4',7'',4'''-O-methyl-amentoflavone | CHEMBL37851...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323212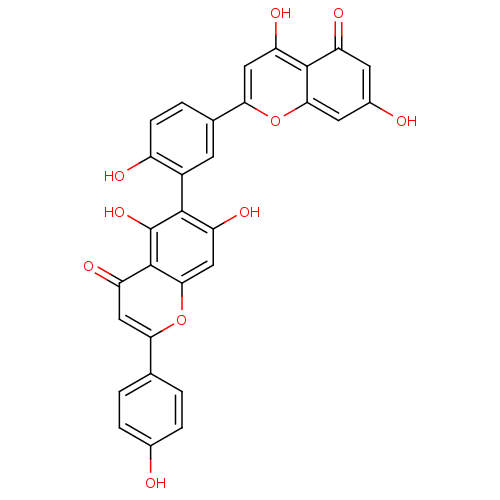 (6-[5-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323207 (CHEMBL1208904 | heveaflavone) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323202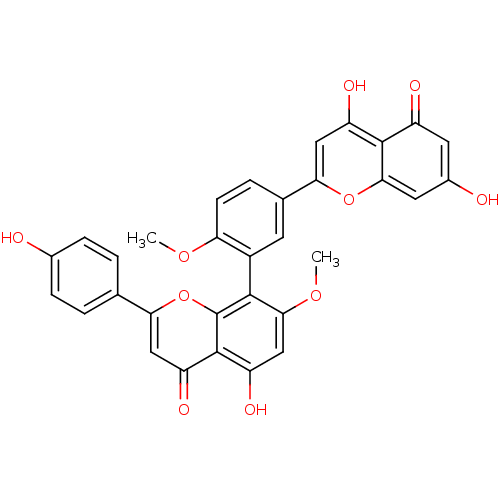 (4',7''-O-methylamentoflavone | 4',7''-di-O-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323204 (7'',4'''-dimethylamentoflavone | CHEMBL374055) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323214 (6-[4-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-phenoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50323205 (7,7'',4'-tri-O-methylamentoflavone | 7-O-methyl-is...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shujitsu University Curated by ChEMBL | Assay Description Inhibition of BACE1 after 60 mins by FRET assay | Bioorg Med Chem Lett 20: 4558-60 (2010) Article DOI: 10.1016/j.bmcl.2010.06.021 BindingDB Entry DOI: 10.7270/Q2GT5P46 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50323203 (CHEMBL1208903 | isoginkgetin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Korea/01310/2001 (H9N2)) neuraminidase after 30 mins by spectrofluorimetric analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM5025 (Oseltamivir | US10919856, POSITIVE CONTROL | ethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50522694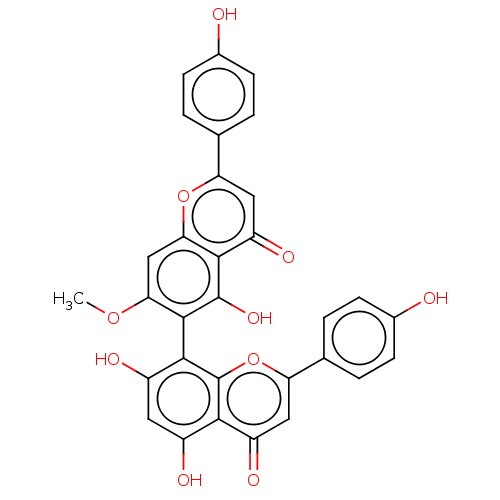 (CHEMBL4449738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50323199 (5,7-dihydroxy-8-(5-(5-hydroxy-7-methoxy-4-oxo-4H-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50522701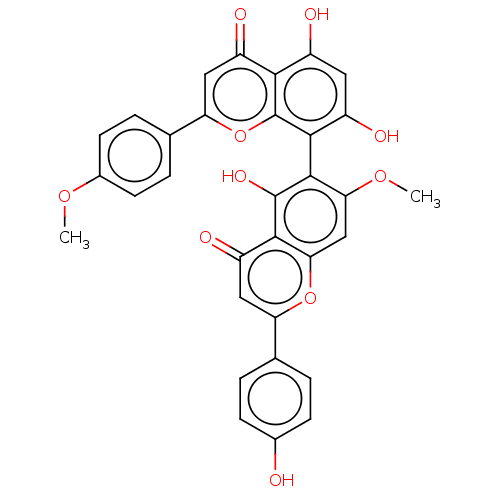 (CHEMBL4554693) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50522697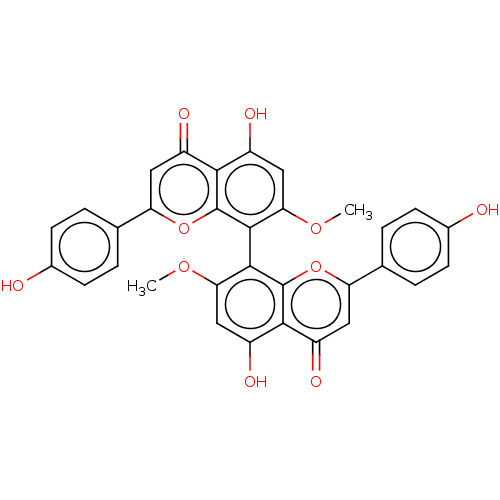 (CHEMBL4463033) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482872 (CHEBI:70655 | CHEMBL1271157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Korea/01310/2001 (H9N2)) neuraminidase after 30 mins by spectrofluorimetric analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50323204 (7'',4'''-dimethylamentoflavone | CHEMBL374055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482872 (CHEBI:70655 | CHEMBL1271157) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Sw/Kor/CAH1/04 (H1N1) KCTC 11165BP neuraminidase after 30 mins by spectrofluorimetric analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50522699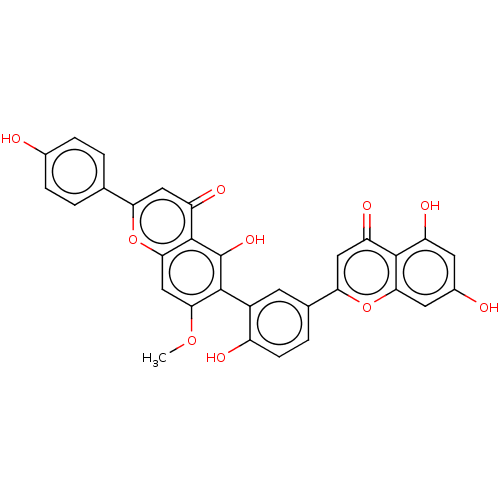 (CHEMBL3589107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human amyloid beta (1 to 40) assessed as reduction in aggregation measured after 24 hrs by ThT fluorescence assay | Bioorg Med Chem Lett 29: 1994-1997 (2019) Article DOI: 10.1016/j.bmcl.2019.05.020 BindingDB Entry DOI: 10.7270/Q2Q81HGR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482882 (CHEBI:70658 | CHEMBL463095) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Korea/01310/2001 (H9N2)) neuraminidase after 30 mins by spectrofluorimetric analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482871 (CHEBI:66265 | CHEMBL509947) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/chicken/Korea/01310/2001 (H9N2)) neuraminidase after 30 mins by spectrofluorimetric analysis | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50482873 (CHEBI:70659 | CHEMBL1271362) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chosun University Curated by ChEMBL | Assay Description Inhibition of oseltamivir-resistant Influenza A virus (A/California/08/2009(H1N1)) neuraminidase H274Y mutant expressed in human 293T cells after 30 ... | J Nat Prod 73: 1636-42 (2010) Article DOI: 10.1021/np1002753 BindingDB Entry DOI: 10.7270/Q2DZ0C4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 157 total ) | Next | Last >> |