 Found 265 hits with Last Name = 'keaney' and Initial = 'ep'
Found 265 hits with Last Name = 'keaney' and Initial = 'ep' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520437
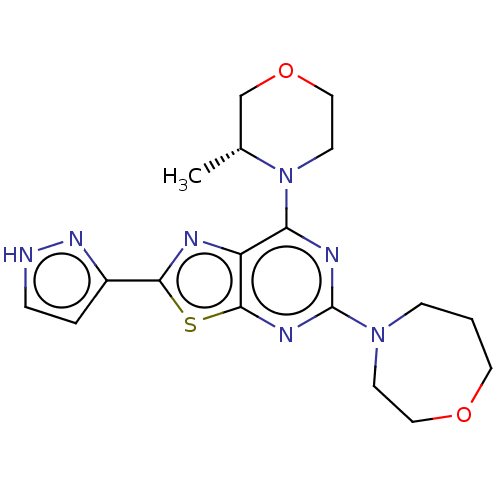
(CHEMBL4562597)Show SMILES C[C@@H]1COCCN1c1nc(nc2sc(nc12)-c1cc[nH]n1)N1CCCOCC1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-12-11-27-10-7-25(12)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)24-5-2-8-26-9-6-24/h3-4,12H,2,5-11H2,1H3,(H,19,23)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520427

(CHEMBL4470066)Show SMILES CC(C)c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C16H20N6OS/c1-9(2)13-19-14(22-6-7-23-8-10(22)3)12-16(20-13)24-15(18-12)11-4-5-17-21-11/h4-5,9-10H,6-8H2,1-3H3,(H,17,21)/t10-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520433

(CHEMBL4451234)Show SMILES C[C@@H]1COCCN1c1nc(nc2sc(nc12)-c1cc[nH]n1)N1CCOCC1 |r| Show InChI InChI=1S/C17H21N7O2S/c1-11-10-26-9-6-24(11)14-13-16(27-15(19-13)12-2-3-18-22-12)21-17(20-14)23-4-7-25-8-5-23/h2-3,11H,4-10H2,1H3,(H,18,22)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520435

(CHEMBL4442382)Show SMILES C[C@@H]1COCCN1c1nc(nc2sc(nc12)-c1cc[nH]n1)C1CCOCC1 |r| Show InChI InChI=1S/C18H22N6O2S/c1-11-10-26-9-6-24(11)16-14-18(27-17(20-14)13-2-5-19-23-13)22-15(21-16)12-3-7-25-8-4-12/h2,5,11-12H,3-4,6-10H2,1H3,(H,19,23)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520430
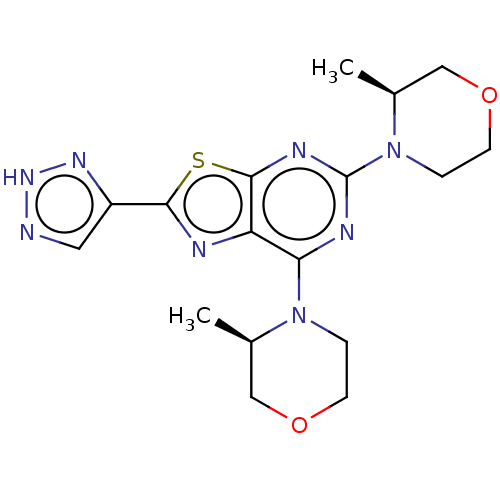
(CHEMBL4515125)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cn[nH]n1 |r| Show InChI InChI=1S/C17H22N8O2S/c1-10-8-26-5-3-24(10)14-13-16(28-15(19-13)12-7-18-23-22-12)21-17(20-14)25-4-6-27-9-11(25)2/h7,10-11H,3-6,8-9H2,1-2H3,(H,18,22,23)/t10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520431

(CHEMBL4566380)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1c[nH]cn1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-8-26-5-3-24(11)15-14-17(28-16(21-14)13-7-19-10-20-13)23-18(22-15)25-4-6-27-9-12(25)2/h7,10-12H,3-6,8-9H2,1-2H3,(H,19,20)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Rattus norvegicus) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR L1460P mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Rattus norvegicus) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR S2215Y mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156302
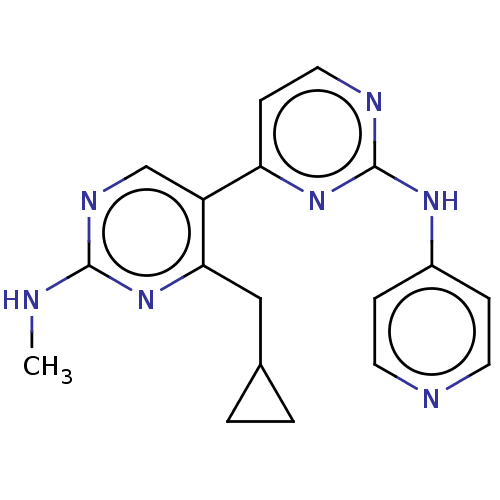
(CHEMBL3780029)Show InChI InChI=1S/C18H19N7/c1-19-17-22-11-14(16(25-17)10-12-2-3-12)15-6-9-21-18(24-15)23-13-4-7-20-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,22,25)(H,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Rattus norvegicus) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot analysis (Rvb = 19... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Rattus norvegicus) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR S2215F mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156294

(CHEMBL3781415)Show InChI InChI=1S/C17H17N7/c18-16-21-10-13(15(23-16)9-11-1-2-11)14-5-8-20-17(24-14)22-12-3-6-19-7-4-12/h3-8,10-11H,1-2,9H2,(H2,18,21,23)(H,19,20,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine-protein kinase ATM
(Homo sapiens (Human)) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ATM (unknown origin) assessed as reduction in CHK2 phosphorylation by cell based assay |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Rattus norvegicus) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR E2419K mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Rattus norvegicus) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR L2427P mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327976

(CHEMBL215476 | N-benzyl-N-(2-((4-cyanophenyl)((1-m...)Show SMILES Cn1cncc1CN(CCN(Cc1ccccc1)S(=O)(=O)c1ccccn1)c1ccc(cc1)C#N Show InChI InChI=1S/C26H26N6O2S/c1-30-21-28-18-25(30)20-31(24-12-10-22(17-27)11-13-24)15-16-32(19-23-7-3-2-4-8-23)35(33,34)26-9-5-6-14-29-26/h2-14,18,21H,15-16,19-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156296

(CHEMBL3781466)Show SMILES CC(C)(O)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7O/c1-21(2,29)13-25-19-24-12-16(18(28-19)11-14-3-4-14)17-7-10-23-20(27-17)26-15-5-8-22-9-6-15/h5-10,12,14,29H,3-4,11,13H2,1-2H3,(H,24,25,28)(H,22,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 in human U2OS cells incubated for 2 hrs by GFP-FYVE reporter gene assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327983

(1-methyl-1H-imidazole-4-sulfonic acid {2-[(4-cyano...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)CCn1cccc1 Show InChI InChI=1S/C24H28N8O2S/c1-28-18-24(27-20-28)35(33,34)32(13-11-30-9-3-4-10-30)14-12-31(17-23-16-26-19-29(23)2)22-7-5-21(15-25)6-8-22/h3-10,16,18-20H,11-14,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327977
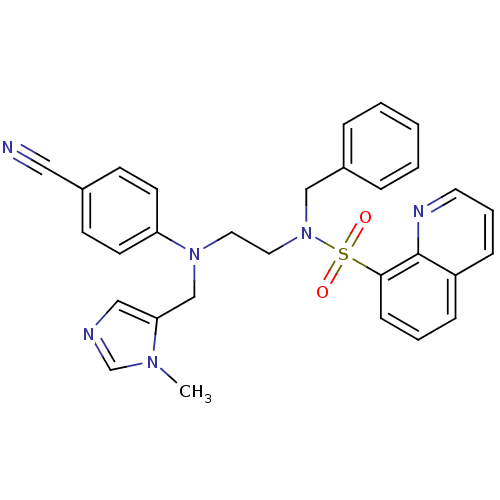
(CHEMBL379511 | N-benzyl-N-(2-((4-cyanophenyl)((1-m...)Show SMILES Cn1cncc1CN(CCN(Cc1ccccc1)S(=O)(=O)c1cccc2cccnc12)c1ccc(cc1)C#N Show InChI InChI=1S/C30H28N6O2S/c1-34-23-32-20-28(34)22-35(27-14-12-24(19-31)13-15-27)17-18-36(21-25-7-3-2-4-8-25)39(37,38)29-11-5-9-26-10-6-16-33-30(26)29/h2-16,20,23H,17-18,21-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156298

(CHEMBL3781926)Show SMILES CC(C)(C)Nc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C21H25N7/c1-21(2,3)28-20-24-13-16(18(27-20)12-14-4-5-14)17-8-11-23-19(26-17)25-15-6-9-22-10-7-15/h6-11,13-14H,4-5,12H2,1-3H3,(H,24,27,28)(H,22,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156302

(CHEMBL3780029)Show InChI InChI=1S/C18H19N7/c1-19-17-22-11-14(16(25-17)10-12-2-3-12)15-6-9-21-18(24-15)23-13-4-7-20-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,22,25)(H,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Rattus norvegicus) | BDBM50520428

(CHEMBL4518369)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-7-5-24(11)15-14-17(28-16(20-14)13-3-4-19-23-13)22-18(21-15)25-6-8-27-10-12(25)2/h3-4,11-12H,5-10H2,1-2H3,(H,19,23)/t11-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR C1483Y mutant in rat primary cortical neuron assessed as reduction in PS6 phosphorylation incubated for 4 hrs by Western blot anal... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520426

(CHEMBL4514031)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc([nH]c2n1)-c1cccc2[nH]ccc12 |r| Show InChI InChI=1S/C23H27N7O2/c1-14-12-31-10-8-29(14)22-19-21(27-23(28-22)30-9-11-32-13-15(30)2)26-20(25-19)17-4-3-5-18-16(17)6-7-24-18/h3-7,14-15,24H,8-13H2,1-2H3,(H,25,26,27,28)/t14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse TSC1-/- MEF cells assessed as inhibition of S6 Ser240/244 phosphorylation incubated for 2 hrs by fluorescence based assay |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13313
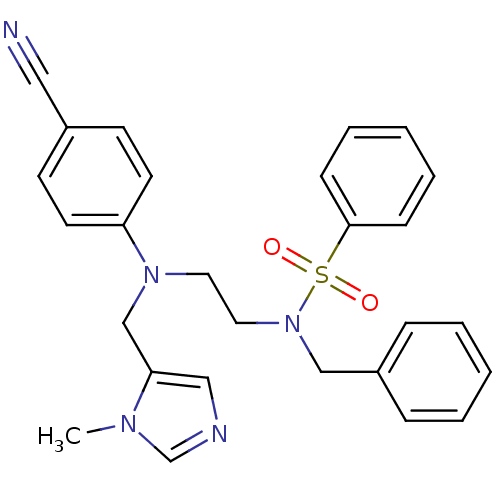
(N-Benzyl-N-{2-[(4-cyanophenyl)-(3-methyl-3H-imidaz...)Show SMILES Cn1cncc1CN(CCN(Cc1ccccc1)S(=O)(=O)c1ccccc1)c1ccc(cc1)C#N Show InChI InChI=1S/C27H27N5O2S/c1-30-22-29-19-26(30)21-31(25-14-12-23(18-28)13-15-25)16-17-32(20-24-8-4-2-5-9-24)35(33,34)27-10-6-3-7-11-27/h2-15,19,22H,16-17,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327974

(CHEMBL1258797 | N-Benzyl,N-{2-[(4-Cyanophenyl)-(3-...)Show SMILES CCCS(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)Cc1ccccc1 Show InChI InChI=1S/C24H29N5O2S/c1-3-15-32(30,31)29(18-22-7-5-4-6-8-22)14-13-28(19-24-17-26-20-27(24)2)23-11-9-21(16-25)10-12-23/h4-12,17,20H,3,13-15,18-19H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327975
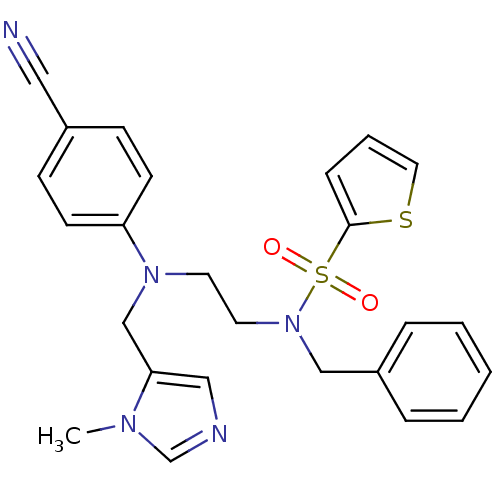
(CHEMBL215475 | N-benzyl-N-(2-((4-cyanophenyl)((1-m...)Show SMILES Cn1cncc1CN(CCN(Cc1ccccc1)S(=O)(=O)c1cccs1)c1ccc(cc1)C#N Show InChI InChI=1S/C25H25N5O2S2/c1-28-20-27-17-24(28)19-29(23-11-9-21(16-26)10-12-23)13-14-30(18-22-6-3-2-4-7-22)34(31,32)25-8-5-15-33-25/h2-12,15,17,20H,13-14,18-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13311
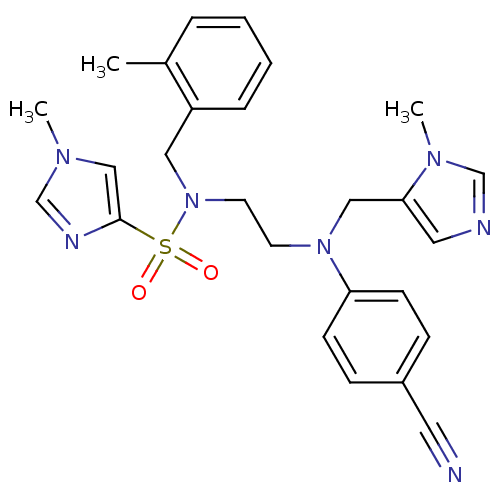
(1-Methyl-1H-imidazole-4-sulfonic Acid {2-[(4-Cyano...)Show SMILES Cc1ccccc1CN(CCN(Cc1cncn1C)c1ccc(cc1)C#N)S(=O)(=O)c1cn(C)cn1 Show InChI InChI=1S/C26H29N7O2S/c1-21-6-4-5-7-23(21)16-33(36(34,35)26-18-30(2)20-29-26)13-12-32(17-25-15-28-19-31(25)3)24-10-8-22(14-27)9-11-24/h4-11,15,18-20H,12-13,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327990
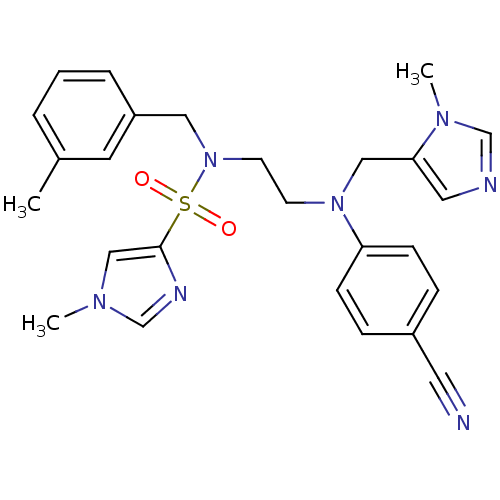
(1-methyl-1H-imidazole-4-sulfonic acid {2-[(4-cyano...)Show SMILES Cc1cccc(CN(CCN(Cc2cncn2C)c2ccc(cc2)C#N)S(=O)(=O)c2cn(C)cn2)c1 Show InChI InChI=1S/C26H29N7O2S/c1-21-5-4-6-23(13-21)16-33(36(34,35)26-18-30(2)20-29-26)12-11-32(17-25-15-28-19-31(25)3)24-9-7-22(14-27)8-10-24/h4-10,13,15,18-20H,11-12,16-17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327979
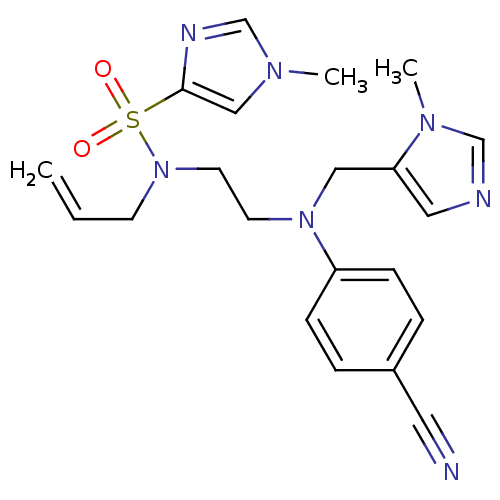
(1-methyl-1H-imidazole-4-sulfonic acid allyl-{2-[(4...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)CC=C Show InChI InChI=1S/C21H25N7O2S/c1-4-9-28(31(29,30)21-15-25(2)17-24-21)11-10-27(14-20-13-23-16-26(20)3)19-7-5-18(12-22)6-8-19/h4-8,13,15-17H,1,9-11,14H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520438
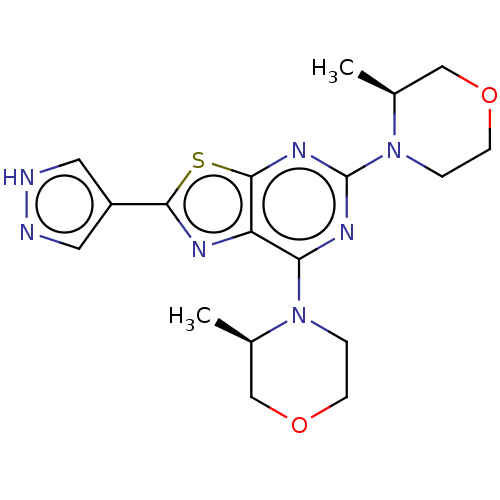
(CHEMBL4460774)Show SMILES C[C@H]1COCCN1c1nc(N2CCOC[C@H]2C)c2nc(sc2n1)-c1cn[nH]c1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-11-9-26-5-3-24(11)15-14-17(28-16(21-14)13-7-19-20-8-13)23-18(22-15)25-4-6-27-10-12(25)2/h7-8,11-12H,3-6,9-10H2,1-2H3,(H,19,20)/t11-,12+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13307

(1-Methyl-1H-imidazole-4-sulfonic Acid Benzyl-{2-[(...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)Cc1ccccc1 Show InChI InChI=1S/C25H27N7O2S/c1-29-18-25(28-20-29)35(33,34)32(16-22-6-4-3-5-7-22)13-12-31(17-24-15-27-19-30(24)2)23-10-8-21(14-26)9-11-23/h3-11,15,18-20H,12-13,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13315

(1-Methyl-1H-imidazole-4-sulfonic Acid {2-[(4-Cyano...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)CC1CCCCC1 Show InChI InChI=1S/C25H33N7O2S/c1-29-18-25(28-20-29)35(33,34)32(16-22-6-4-3-5-7-22)13-12-31(17-24-15-27-19-30(24)2)23-10-8-21(14-26)9-11-23/h8-11,15,18-20,22H,3-7,12-13,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50134041
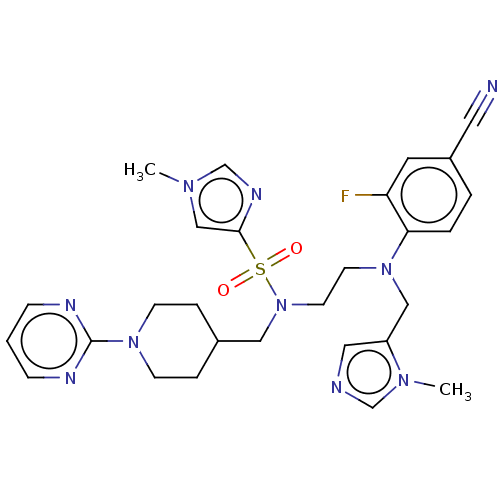
(CHEMBL1256439)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1F)C#N)CC1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C28H33FN10O2S/c1-35-19-27(34-21-35)42(40,41)39(17-22-6-10-37(11-7-22)28-32-8-3-9-33-28)13-12-38(18-24-16-31-20-36(24)2)26-5-4-23(15-30)14-25(26)29/h3-5,8-9,14,16,19-22H,6-7,10-13,17-18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327985
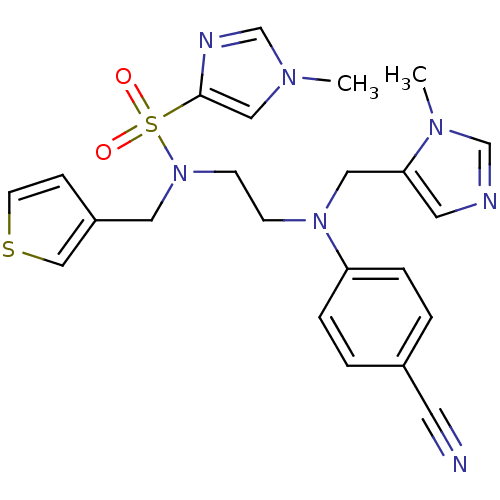
(CHEMBL452319 | N-(2-((4-cyanophenyl)((1-methyl-1H-...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)Cc1ccsc1 Show InChI InChI=1S/C23H25N7O2S2/c1-27-15-23(26-18-27)34(31,32)30(13-20-7-10-33-16-20)9-8-29(14-22-12-25-17-28(22)2)21-5-3-19(11-24)4-6-21/h3-7,10,12,15-18H,8-9,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50327974

(CHEMBL1258797 | N-Benzyl,N-{2-[(4-Cyanophenyl)-(3-...)Show SMILES CCCS(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)Cc1ccccc1 Show InChI InChI=1S/C24H29N5O2S/c1-3-15-32(30,31)29(18-22-7-5-4-6-8-22)14-13-28(19-24-17-26-20-27(24)2)23-11-9-21(16-25)10-12-23/h4-12,17,20H,3,13-15,18-19H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of H-Ras farnesylation expressed in mouse NIH3T3 cells |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327982

(1-methyl-1H-imidazole-4-sulfonic acid {2-[(4-cyano...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)Cc1ccncc1 Show InChI InChI=1S/C24H26N8O2S/c1-29-17-24(28-19-29)35(33,34)32(15-21-7-9-26-10-8-21)12-11-31(16-23-14-27-18-30(23)2)22-5-3-20(13-25)4-6-22/h3-10,14,17-19H,11-12,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156295
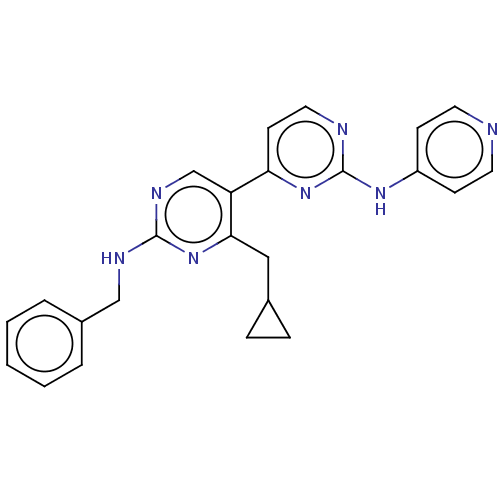
(CHEMBL3781615)Show SMILES C(Nc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1)c1ccccc1 Show InChI InChI=1S/C24H23N7/c1-2-4-18(5-3-1)15-27-23-28-16-20(22(31-23)14-17-6-7-17)21-10-13-26-24(30-21)29-19-8-11-25-12-9-19/h1-5,8-13,16-17H,6-7,14-15H2,(H,27,28,31)(H,25,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50327992
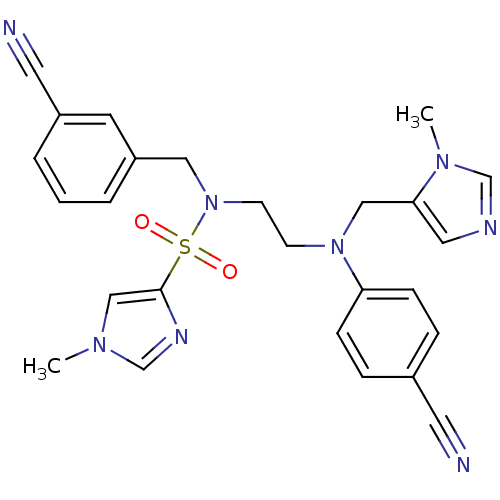
(1-methyl-1H-imidazole-4-sulfonic acid (3-cyanobenz...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)Cc1cccc(c1)C#N Show InChI InChI=1S/C26H26N8O2S/c1-31-18-26(30-20-31)37(35,36)34(16-23-5-3-4-22(12-23)14-28)11-10-33(17-25-15-29-19-32(25)2)24-8-6-21(13-27)7-9-24/h3-9,12,15,18-20H,10-11,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM13308

(N-benzyl-N-(2-{(4-bromophenyl)[(1-methyl-1H-imidaz...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(Br)cc1)Cc1ccccc1 Show InChI InChI=1S/C24H27BrN6O2S/c1-28-17-24(27-19-28)34(32,33)31(15-20-6-4-3-5-7-20)13-12-30(16-23-14-26-18-29(23)2)22-10-8-21(25)9-11-22/h3-11,14,17-19H,12-13,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50133900

(CHEMBL1258798)Show SMILES Cn1cncc1CN(CCN(Cc1ccccc1)S(=O)(=O)C1CC1)c1ccc(cc1)C#N Show InChI InChI=1S/C24H27N5O2S/c1-27-19-26-16-23(27)18-28(22-9-7-20(15-25)8-10-22)13-14-29(32(30,31)24-11-12-24)17-21-5-3-2-4-6-21/h2-10,16,19,24H,11-14,17-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of human farnesyltransferase assessed as incorporation of [3H]FPP into H-Ras-CVLS |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156297
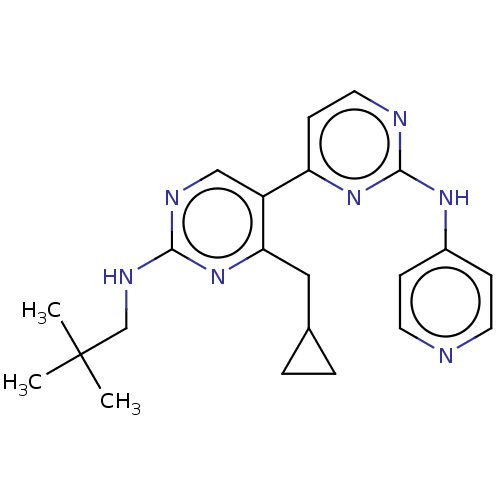
(CHEMBL3780339)Show SMILES CC(C)(C)CNc1ncc(c(CC2CC2)n1)-c1ccnc(Nc2ccncc2)n1 Show InChI InChI=1S/C22H27N7/c1-22(2,3)14-26-20-25-13-17(19(29-20)12-15-4-5-15)18-8-11-24-21(28-18)27-16-6-9-23-10-7-16/h6-11,13,15H,4-5,12,14H2,1-3H3,(H,25,26,29)(H,23,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50327998

(CHEMBL1258350 | N-(2-((4-cyano-2-fluorophenyl)((1-...)Show SMILES Cn1cncc1CN(CCN(CC1CCCCC1)S(=O)(=O)c1ccccn1)c1ccc(cc1F)C#N Show InChI InChI=1S/C26H31FN6O2S/c1-31-20-29-17-23(31)19-32(25-11-10-22(16-28)15-24(25)27)13-14-33(18-21-7-3-2-4-8-21)36(34,35)26-9-5-6-12-30-26/h5-6,9-12,15,17,20-21H,2-4,7-8,13-14,18-19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of H-Ras farnesylation expressed in mouse NIH3T3 cells |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50327976

(CHEMBL215476 | N-benzyl-N-(2-((4-cyanophenyl)((1-m...)Show SMILES Cn1cncc1CN(CCN(Cc1ccccc1)S(=O)(=O)c1ccccn1)c1ccc(cc1)C#N Show InChI InChI=1S/C26H26N6O2S/c1-30-21-28-18-25(30)20-31(24-12-10-22(17-27)11-13-24)15-16-32(19-23-7-3-2-4-8-23)35(33,34)26-9-5-6-14-29-26/h2-14,18,21H,15-16,19-20H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of H-Ras farnesylation expressed in mouse NIH3T3 cells |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156300

(CHEMBL3781634)Show InChI InChI=1S/C18H18N6O/c1-25-18-21-11-14(16(24-18)10-12-2-3-12)15-6-9-20-17(23-15)22-13-4-7-19-8-5-13/h4-9,11-12H,2-3,10H2,1H3,(H,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Mus musculus (Mouse)) | BDBM50520439

(CHEMBL4468727)Show SMILES C[C@@H]1COCCN1c1nc(CN2CCOCC2)nc2sc(nc12)-c1cc[nH]n1 |r| Show InChI InChI=1S/C18H23N7O2S/c1-12-11-27-9-6-25(12)16-15-18(28-17(22-15)13-2-3-19-23-13)21-14(20-16)10-24-4-7-26-8-5-24/h2-3,12H,4-11H2,1H3,(H,19,23)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR in mouse MEF cells harboring TSC1 deletion mutant assessed as reduction in PS6 phosphorylation incuabted for 2 hrs alexa fluor 594... |
J Med Chem 63: 1068-1083 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01398
BindingDB Entry DOI: 10.7270/Q29S1VFW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM13315

(1-Methyl-1H-imidazole-4-sulfonic Acid {2-[(4-Cyano...)Show SMILES Cn1cnc(c1)S(=O)(=O)N(CCN(Cc1cncn1C)c1ccc(cc1)C#N)CC1CCCCC1 Show InChI InChI=1S/C25H33N7O2S/c1-29-18-25(28-20-29)35(33,34)32(16-22-6-4-3-5-7-22)13-12-31(17-24-15-27-19-30(24)2)23-10-8-21(14-26)9-11-23/h8-11,15,18-20,22H,3-7,12-13,16-17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of H-Ras farnesylation expressed in mouse NIH3T3 cells |
J Med Chem 53: 6867-88 (2010)
Article DOI: 10.1021/jm1001748
BindingDB Entry DOI: 10.7270/Q26110JC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50156293
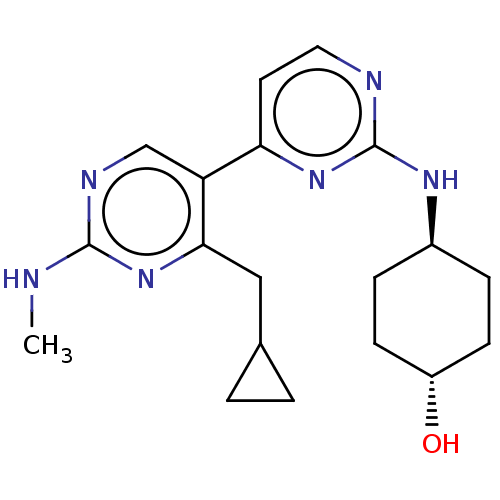
(CHEMBL3781916)Show SMILES CNc1ncc(c(CC2CC2)n1)-c1ccnc(N[C@H]2CC[C@H](O)CC2)n1 |r,wU:21.23,wD:18.19,(-9.3,9.74,;-9.31,8.51,;-7.98,7.73,;-6.64,8.49,;-5.31,7.71,;-5.32,6.17,;-6.66,5.41,;-6.67,3.87,;-8.01,3.11,;-8.71,1.83,;-9.47,3.17,;-7.99,6.19,;-3.99,5.4,;-2.65,6.16,;-1.32,5.39,;-1.33,3.85,;-2.66,3.08,;-2.67,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;2.4,-1.39,;1.33,.77,;,1.54,;-4,3.86,)| Show InChI InChI=1S/C19H26N6O/c1-20-18-22-11-15(17(25-18)10-12-2-3-12)16-8-9-21-19(24-16)23-13-4-6-14(26)7-5-13/h8-9,11-14,26H,2-7,10H2,1H3,(H,20,22,25)(H,21,23,24)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human VPS34 using L-alpha-phosphatidylinositol as substrate incubated for 10 mins by luminescence based ATP detection assay |
ACS Med Chem Lett 7: 72-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00335
BindingDB Entry DOI: 10.7270/Q2K35WK6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data