

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278990 (CHEMBL4164524) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278986 (CHEMBL4161860) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278968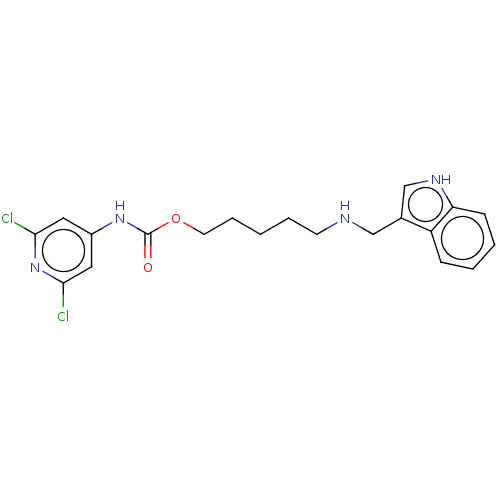 (CHEMBL4164186) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278987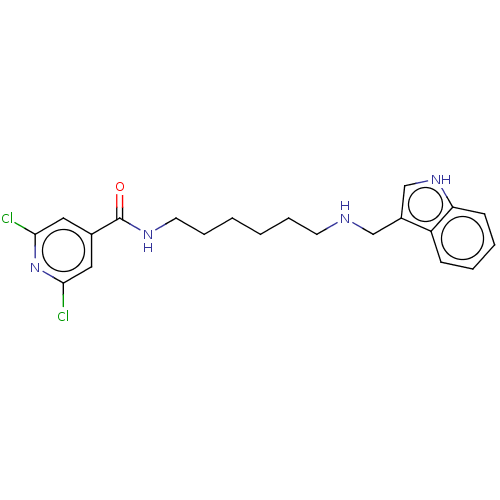 (CHEMBL4165299) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278969 (CHEMBL4160790) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278984 (CHEMBL4168710) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 288 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278970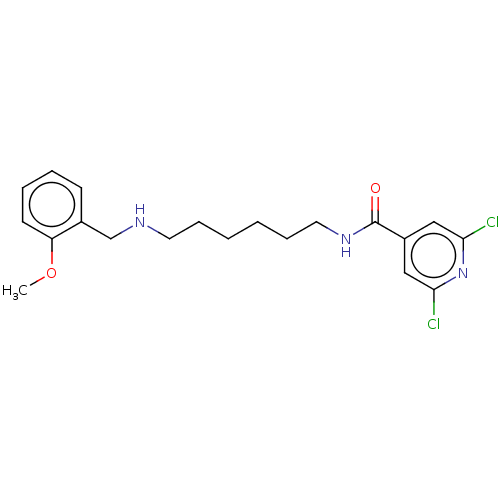 (CHEMBL4175914) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50278983 (CHEMBL4172510) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 404 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Mixed inhibition of electric eel AChE using acetylthiocholine iodide as substrate measured from 0.5 to 1.5 mins by Dixon plot analysis | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of binding to human integrin receptor alpha V beta3 | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278990 (CHEMBL4164524) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 424 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278968 (CHEMBL4164186) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 648 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278969 (CHEMBL4160790) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278968 (CHEMBL4164186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 828 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278986 (CHEMBL4161860) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278969 (CHEMBL4160790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278984 (CHEMBL4168710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278983 (CHEMBL4172510) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278990 (CHEMBL4164524) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278987 (CHEMBL4165299) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278983 (CHEMBL4172510) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278984 (CHEMBL4168710) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278970 (CHEMBL4175914) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278985 (CHEMBL4172060) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278987 (CHEMBL4165299) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278988 (CHEMBL4163781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278986 (CHEMBL4161860) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50278970 (CHEMBL4175914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 mi... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50278988 (CHEMBL4163781) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza University of Rome Curated by ChEMBL | Assay Description Inhibition of human serum AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured for 3 min... | Eur J Med Chem 141: 197-210 (2017) Article DOI: 10.1016/j.ejmech.2017.09.022 BindingDB Entry DOI: 10.7270/Q27083ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||