 Found 295 hits with Last Name = 'lillich' and Initial = 'ff'
Found 295 hits with Last Name = 'lillich' and Initial = 'ff' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594427

(CHEMBL5172496)Show SMILES OC(=O)CCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327809
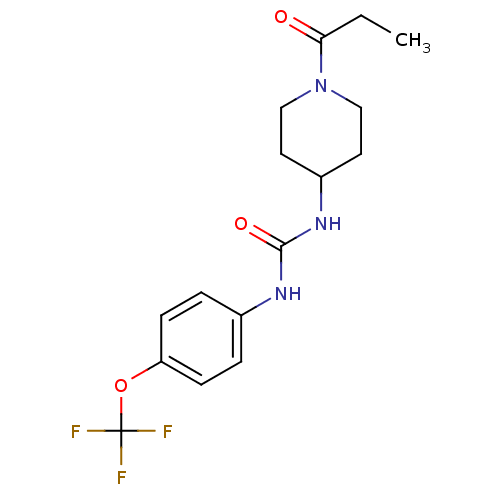
(1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H20F3N3O3/c1-2-14(23)22-9-7-12(8-10-22)21-15(24)20-11-3-5-13(6-4-11)25-16(17,18)19/h3-6,12H,2,7-10H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594426
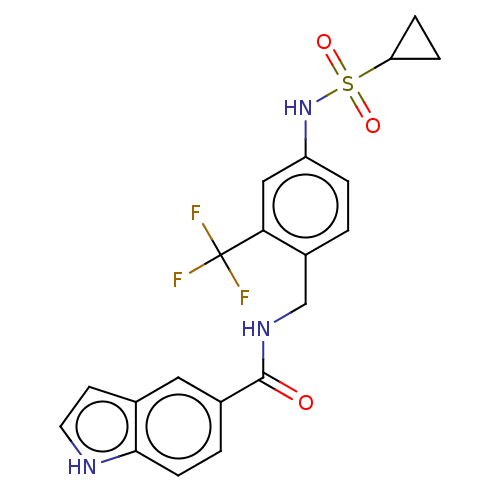
(CHEMBL5177249)Show SMILES FC(F)(F)c1cc(NS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2[nH]ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50530480

(CHEMBL4445524)Show SMILES OC(=O)CCCc1nc(c(o1)-c1ccccc1)-c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H15Cl2NO3/c20-14-10-9-13(11-15(14)21)18-19(12-5-2-1-3-6-12)25-16(22-18)7-4-8-17(23)24/h1-3,5-6,9-11H,4,7-8H2,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of full length human soluble epoxide hydrolase pre-incubated for 30 mins before DiFMUP substrate addition by fluorescence base... |
J Med Chem 62: 8443-8460 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00445
BindingDB Entry DOI: 10.7270/Q2V98CJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594428

(CHEMBL5192349)Show SMILES COc1ccc(CNC(=O)[C@@H]2CCC[C@@H](C2)Nc2ccc([N+]([O-])=O)c3nonc23)c(c1)C(F)(F)F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586969

(CHEMBL5088299)Show SMILES Fc1cccc(c1)S(=O)(=O)n1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586956

(CHEMBL5075652)Show SMILES CS(=O)(=O)Cc1ccc(CNC(=O)c2ccc3n(ccc3c2)S(=O)(=O)c2cccc(F)c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586961

(CHEMBL5078180)Show SMILES CC(C)Cn1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586964

(CHEMBL5074984)Show SMILES CC(C)S(=O)(=O)n1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594427

(CHEMBL5172496)Show SMILES OC(=O)CCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586965

(CHEMBL5074074)Show SMILES COCCn1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586962
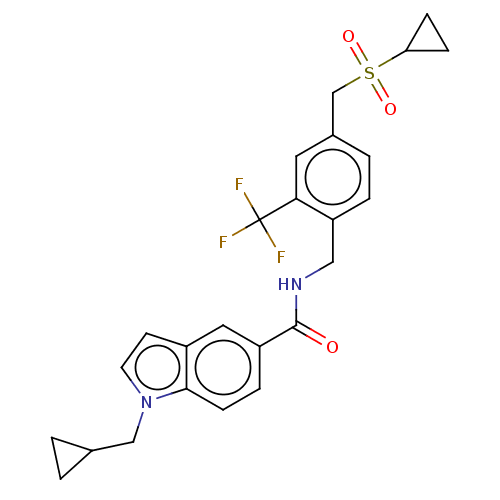
(CHEMBL5081457)Show SMILES FC(F)(F)c1cc(CS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2n(CC3CC3)ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586954
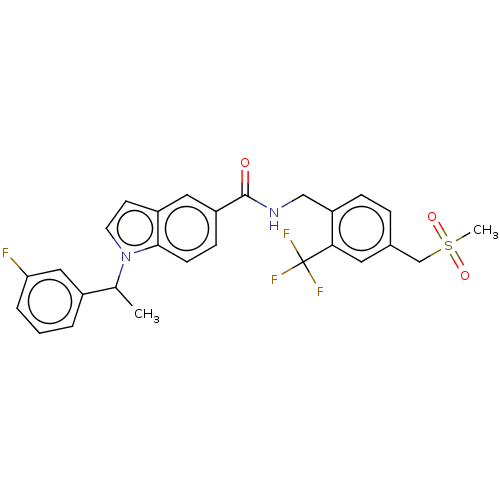
(CHEMBL5079563)Show SMILES CC(c1cccc(F)c1)n1ccc2cc(ccc12)C(=O)NCc1ccc(CS(C)(=O)=O)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586945
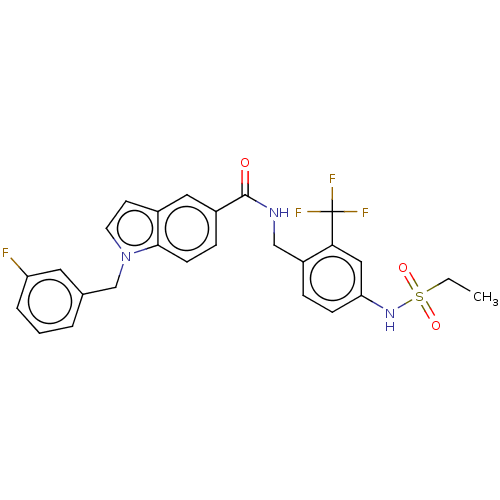
(CHEMBL5084314)Show SMILES CCS(=O)(=O)Nc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ccc3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586963

(CHEMBL5090087)Show SMILES FC(F)(F)C(Cn1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586955

(CHEMBL5090873)Show SMILES CS(=O)(=O)Cc1ccc(CNC(=O)c2ccc3n(ccc3c2)C(=O)c2cccc(F)c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586947

(CHEMBL5087833)Show SMILES Fc1cccc(Cn2ccc3cc(ccc23)C(=O)NCc2ccc(NC(=O)C(F)(F)F)cc2C(F)(F)F)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586960

(CHEMBL5081013)Show SMILES CC(C)n1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586966
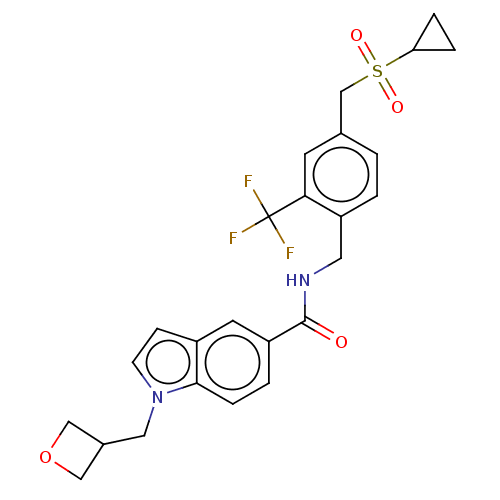
(CHEMBL5091134)Show SMILES FC(F)(F)c1cc(CS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2n(CC3COC3)ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586944

(CHEMBL5090821)Show SMILES CS(=O)(=O)Nc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ccc3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586948
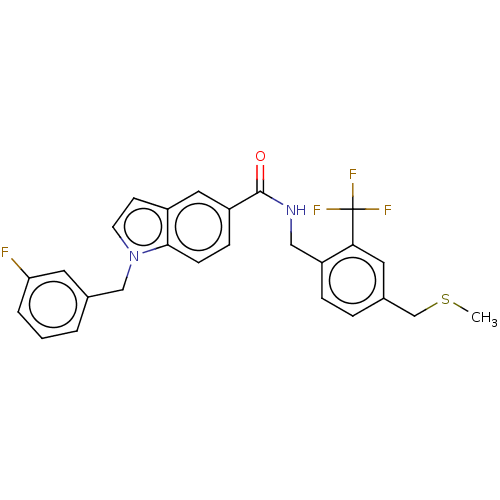
(CHEMBL5087046)Show SMILES CSCc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ccc3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586949
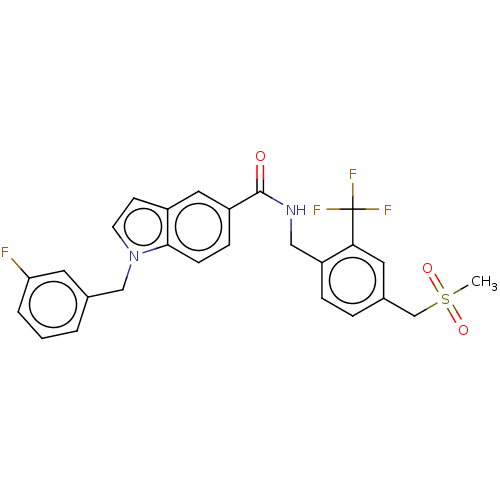
(CHEMBL5080474)Show SMILES CS(=O)(=O)Cc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ccc3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586952
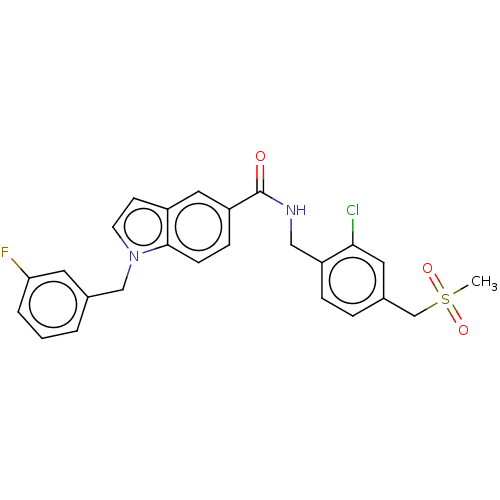
(CHEMBL5094953)Show SMILES CS(=O)(=O)Cc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ccc3c2)c(Cl)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586959
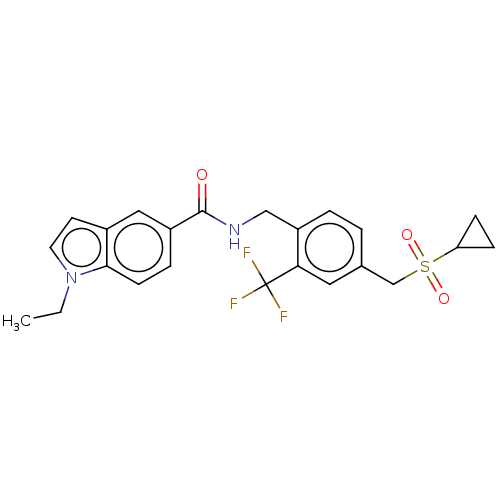
(CHEMBL5092964)Show SMILES CCn1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586943
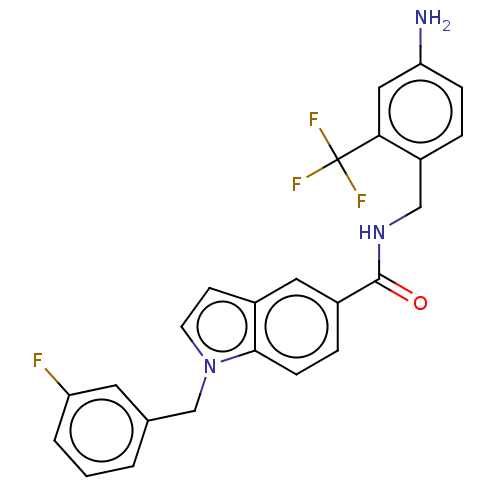
(CHEMBL5087861)Show SMILES Nc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ccc3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50594428

(CHEMBL5192349)Show SMILES COc1ccc(CNC(=O)[C@@H]2CCC[C@@H](C2)Nc2ccc([N+]([O-])=O)c3nonc23)c(c1)C(F)(F)F |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586946
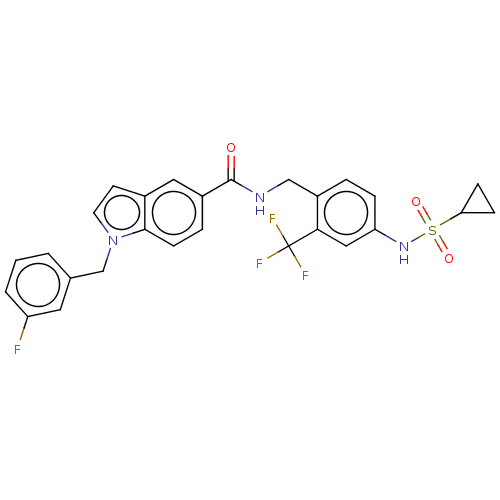
(CHEMBL5084336)Show SMILES Fc1cccc(Cn2ccc3cc(ccc23)C(=O)NCc2ccc(NS(=O)(=O)C3CC3)cc2C(F)(F)F)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50586956

(CHEMBL5075652)Show SMILES CS(=O)(=O)Cc1ccc(CNC(=O)c2ccc3n(ccc3c2)S(=O)(=O)c2cccc(F)c2)c(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His-tagged mouse sEH (2 to 554 residues) expressed in Escherichia coli Rosetta2 (DE3) assessed as reduction in 6-methoxy-2-n... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586951

(CHEMBL5074222)Show SMILES CS(=O)(=O)Cc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ccc3c2)c(F)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586958
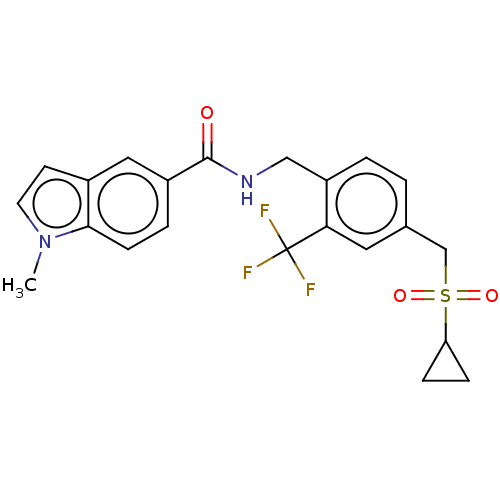
(CHEMBL5090820)Show SMILES Cn1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586971
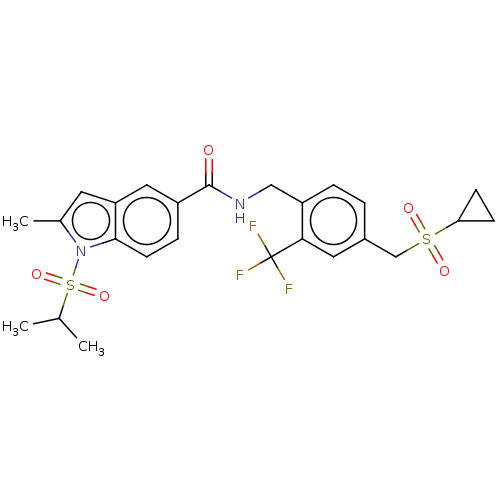
(CHEMBL5076679)Show SMILES CC(C)S(=O)(=O)n1c(C)cc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50327809

(1-(1-Propionylpiperidin-4-yl)-3-(4-(trifluorometho...)Show SMILES CCC(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H20F3N3O3/c1-2-14(23)22-9-7-12(8-10-22)21-15(24)20-11-3-5-13(6-4-11)25-16(17,18)19/h3-6,12H,2,7-10H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586940
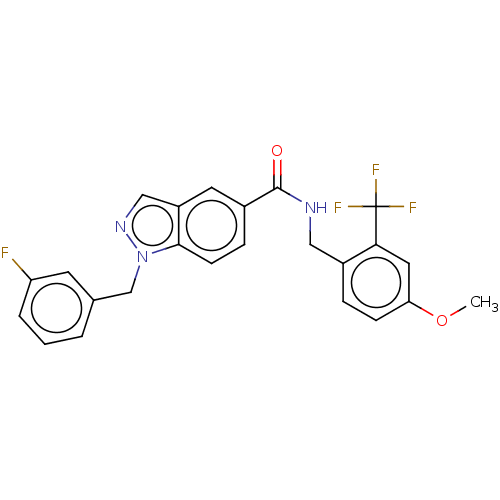
(CHEMBL5092049)Show SMILES COc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ncc3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586967
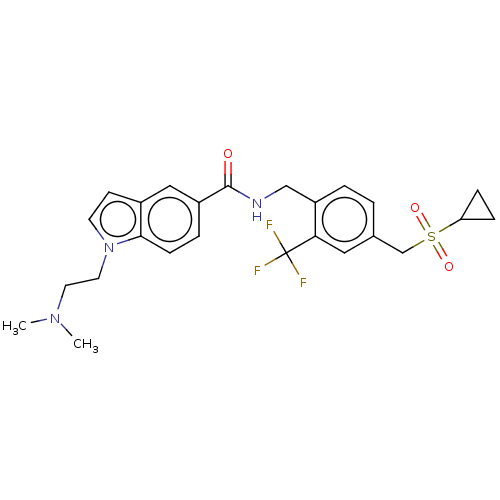
(CHEMBL5076425)Show SMILES CN(C)CCn1ccc2cc(ccc12)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586935

(CHEMBL5087313)Show SMILES COc1ccc(CNC(=O)c2ccc3n(Cc4ccccc4)c(=O)[nH]c3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594426

(CHEMBL5177249)Show SMILES FC(F)(F)c1cc(NS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2[nH]ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586936
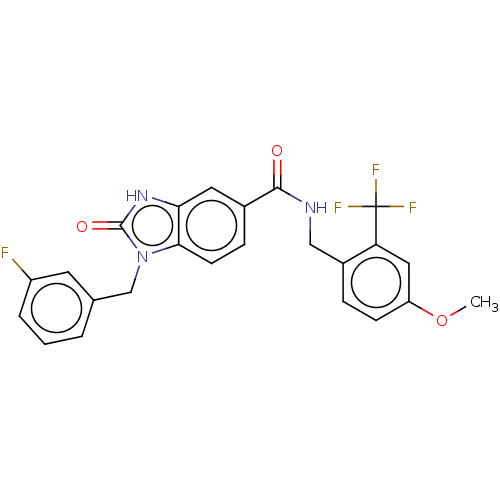
(CHEMBL5080856)Show SMILES COc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)c(=O)[nH]c3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586957

(CHEMBL5077363)Show SMILES FC(F)(F)c1cc(CS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2[nH]ccc2c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50594427

(CHEMBL5172496)Show SMILES OC(=O)CCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:16:17:20:24.23.22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00073
BindingDB Entry DOI: 10.7270/Q2W099ZC |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586970
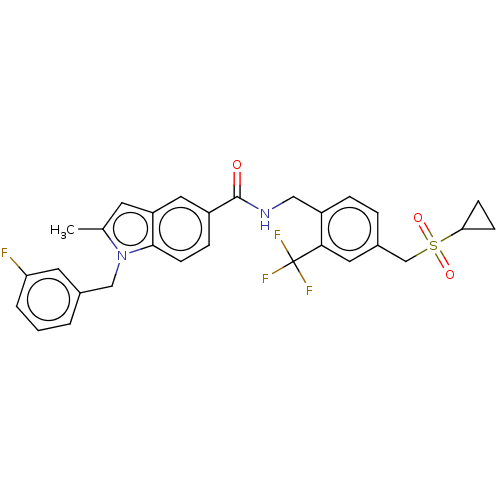
(CHEMBL5074383)Show SMILES Cc1cc2cc(ccc2n1Cc1cccc(F)c1)C(=O)NCc1ccc(CS(=O)(=O)C2CC2)cc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586939
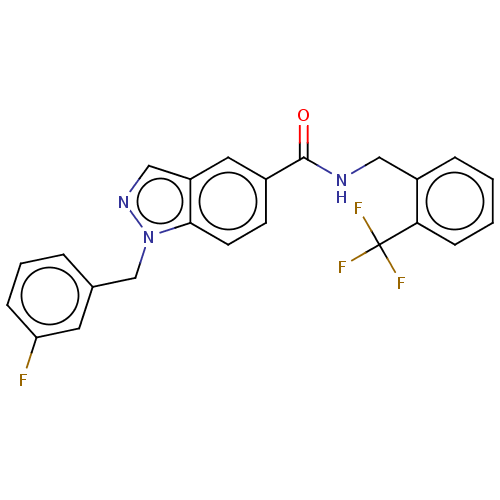
(CHEMBL5080632)Show SMILES Fc1cccc(Cn2ncc3cc(ccc23)C(=O)NCc2ccccc2C(F)(F)F)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586938
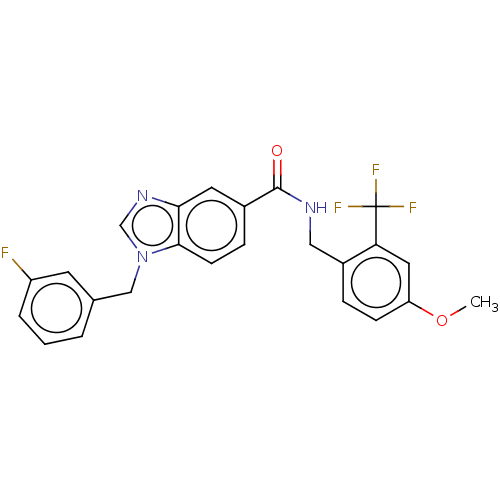
(CHEMBL5082042)Show SMILES COc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)cnc3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586953
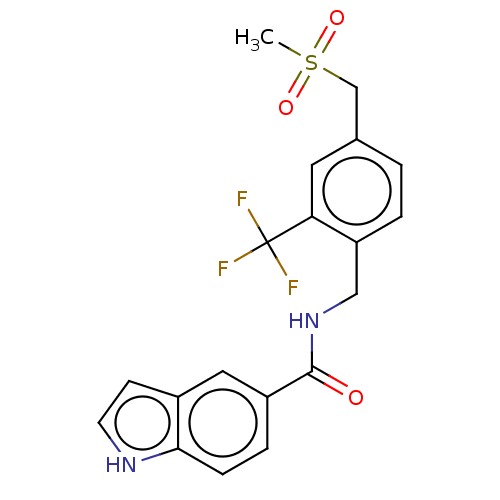
(CHEMBL5085618)Show SMILES CS(=O)(=O)Cc1ccc(CNC(=O)c2ccc3[nH]ccc3c2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50586944

(CHEMBL5090821)Show SMILES CS(=O)(=O)Nc1ccc(CNC(=O)c2ccc3n(Cc4cccc(F)c4)ccc3c2)c(c1)C(F)(F)F | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His-tagged mouse sEH (2 to 554 residues) expressed in Escherichia coli Rosetta2 (DE3) assessed as reduction in 6-methoxy-2-n... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586927
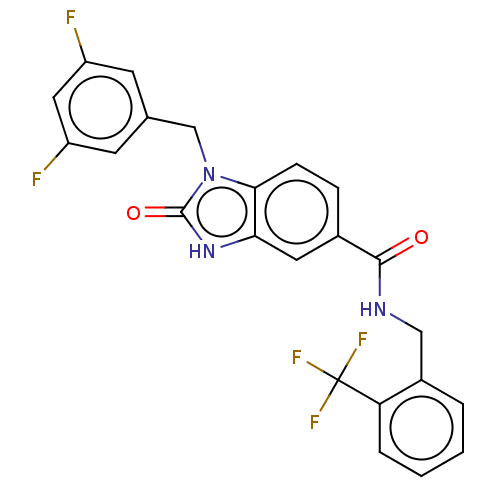
(CHEMBL5075443)Show SMILES Fc1cc(F)cc(Cn2c3ccc(cc3[nH]c2=O)C(=O)NCc2ccccc2C(F)(F)F)c1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50586924
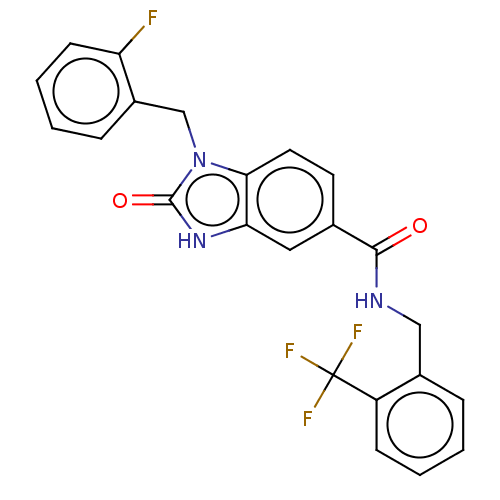
(CHEMBL5093048)Show SMILES Fc1ccccc1Cn1c2ccc(cc2[nH]c1=O)C(=O)NCc1ccccc1C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01331
BindingDB Entry DOI: 10.7270/Q2T43Z05 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data