

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50040523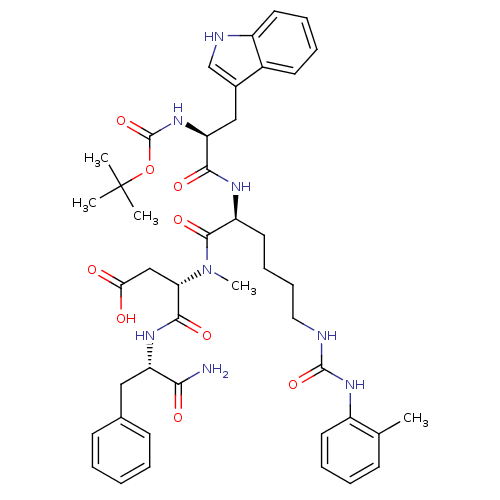 ((S)-3-{[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007925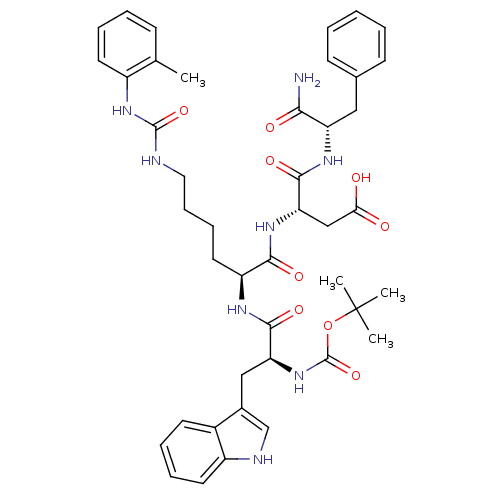 ((S)-3-[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007916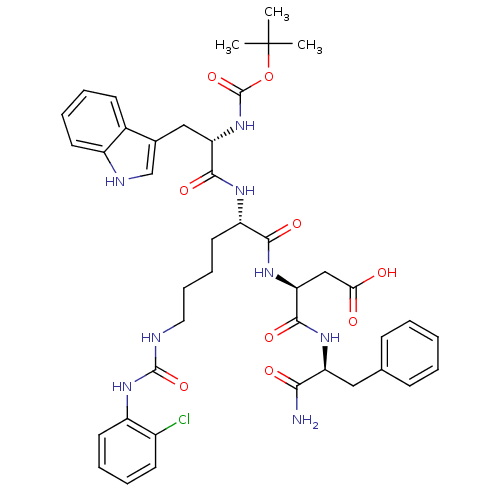 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007917 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007919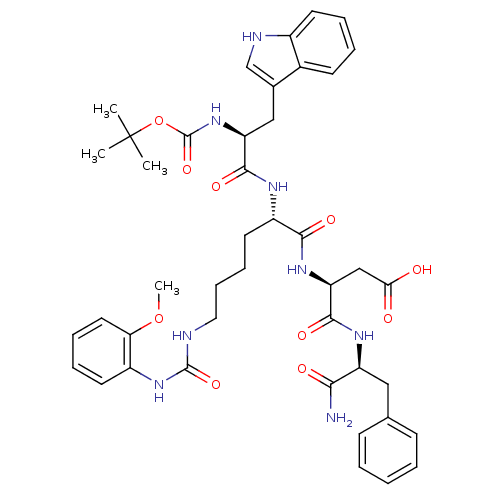 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007918 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007922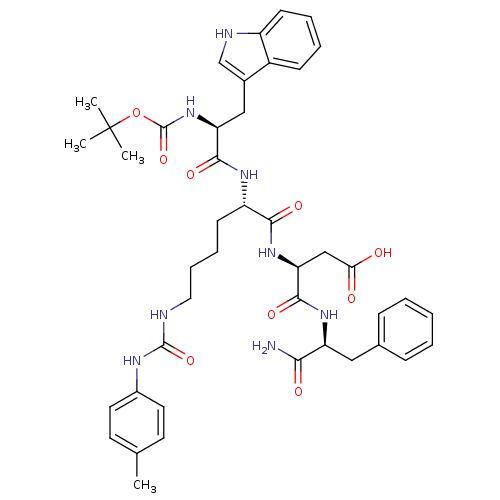 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214078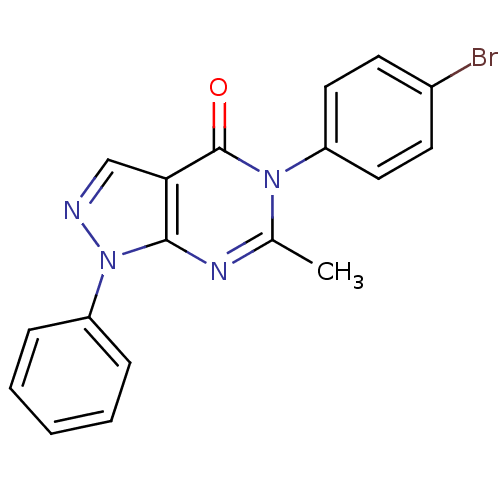 (5-(4-bromophenyl)-6-methyl-1-phenyl-1H-pyrazolo[3,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214076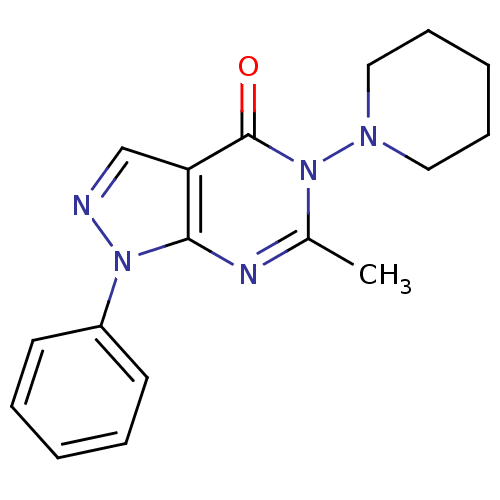 (6-methyl-1-phenyl-5-(piperidin-1-yl)-1H-pyrazolo[3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214074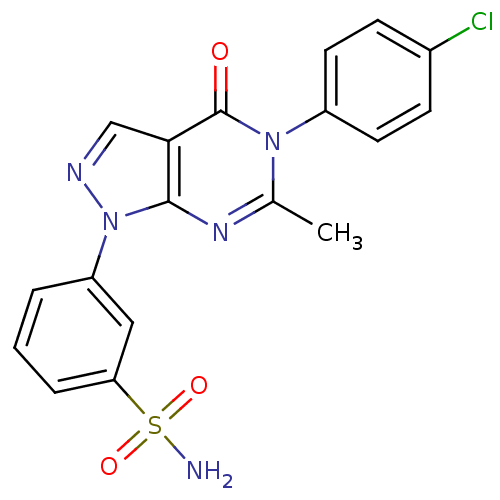 (3-(5-(4-chlorophenyl)-6-methyl-4-oxo-4,5-dihydropy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007921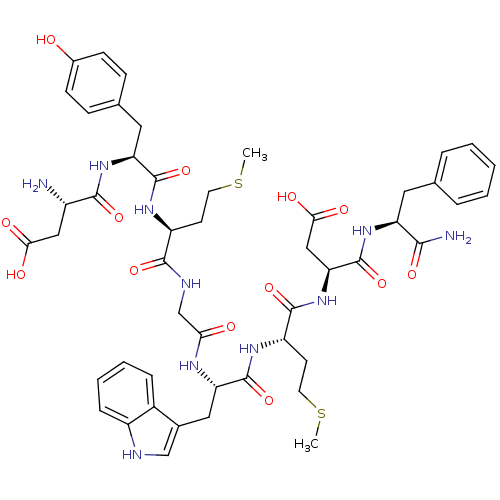 (3-{2-[2-(2-{2-[2-(2-Amino-3-carboxy-propionylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214088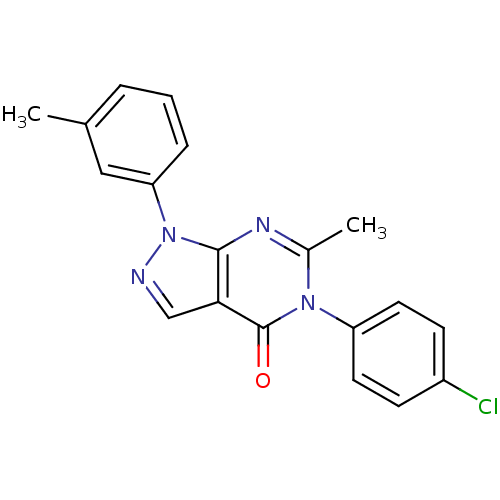 (5-(4-chlorophenyl)-6-methyl-1-m-tolyl-1H-pyrazolo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007921 (3-{2-[2-(2-{2-[2-(2-Amino-3-carboxy-propionylamino...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214079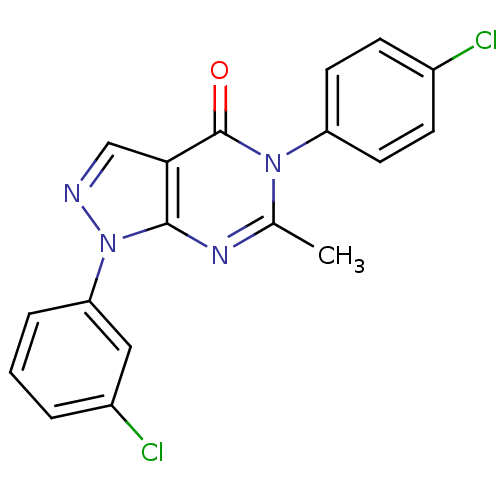 (1-(3-chlorophenyl)-5-(4-chlorophenyl)-6-methyl-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214075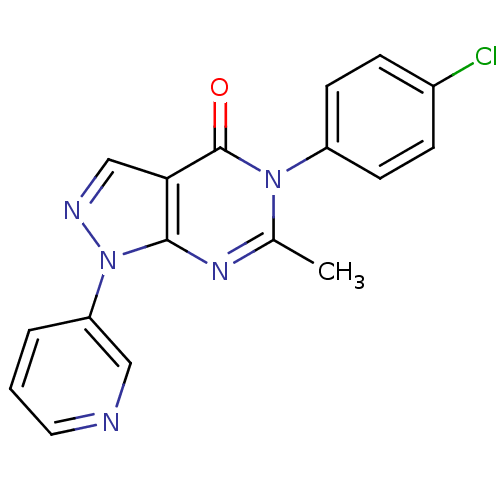 (5-(4-chlorophenyl)-6-methyl-1-(pyridin-3-yl)-1H-py...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214071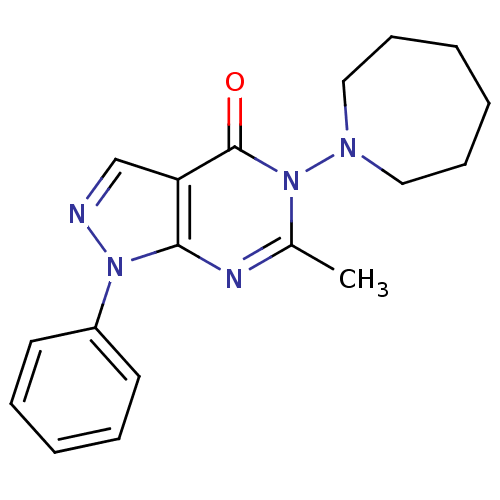 (5-(azepan-1-yl)-6-methyl-1-phenyl-1H-pyrazolo[3,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214073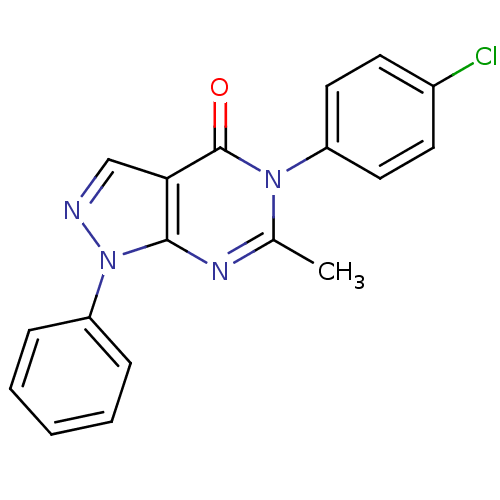 (5-(4-chlorophenyl)-6-methyl-1-phenyl-1H-pyrazolo[3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214089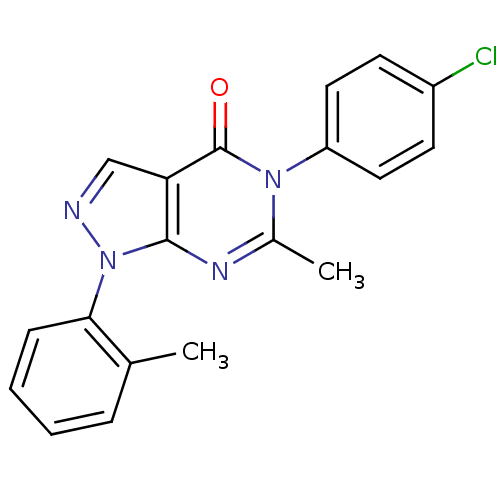 (5-(4-chlorophenyl)-6-methyl-1-o-tolyl-1H-pyrazolo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007923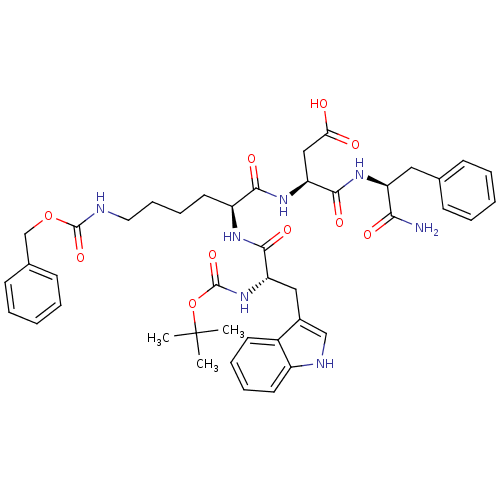 (3-[5-aminocarboxybenzyl-1-[2-(1H-3-indolyl)-1-buty...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214085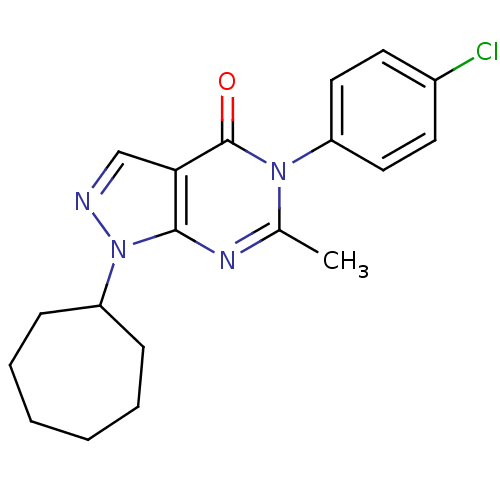 (5-(4-chlorophenyl)-1-cycloheptyl-6-methyl-1H-pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50225106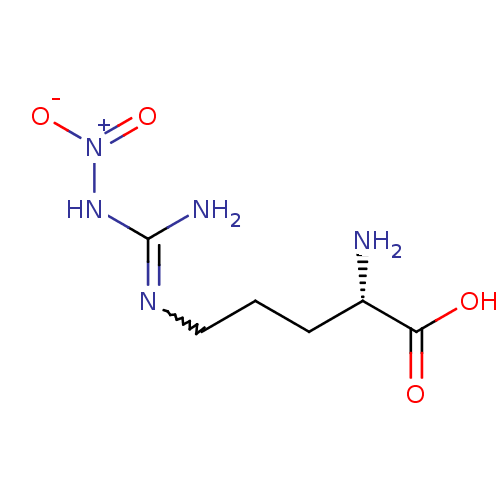 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against isomeric form of Neuronal nitric oxide synthase measured by citrulline assay | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214077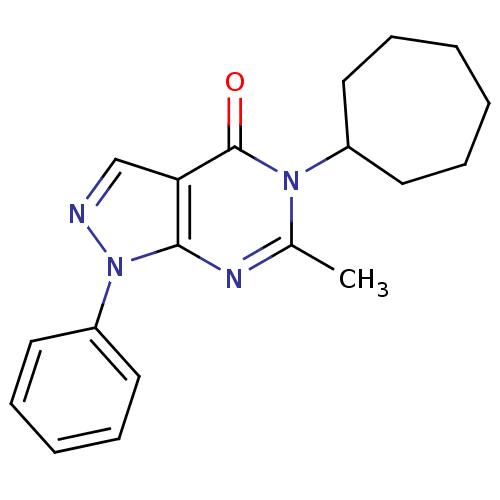 (5-cycloheptyl-6-methyl-1-phenyl-1H-pyrazolo[3,4-d]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214082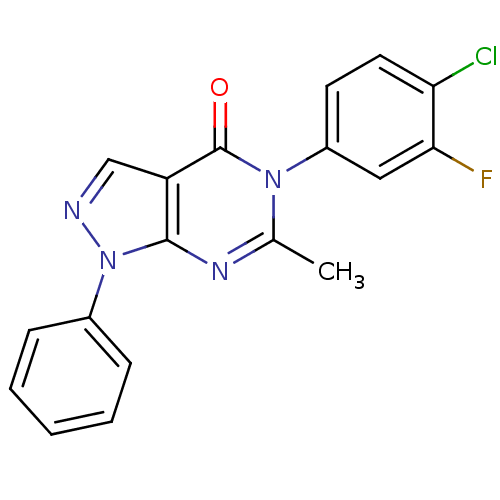 (5-(4-chloro-3-fluorophenyl)-6-methyl-1-phenyl-1H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50007924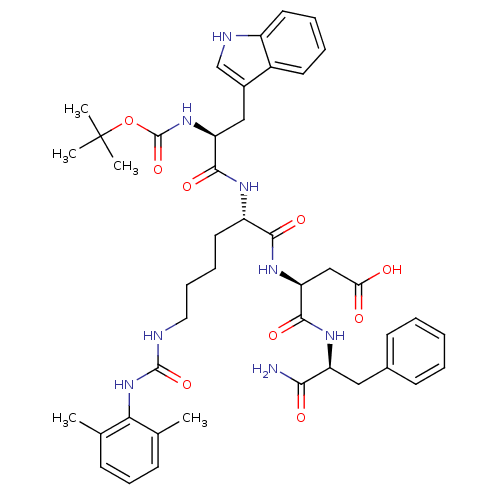 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007918 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214083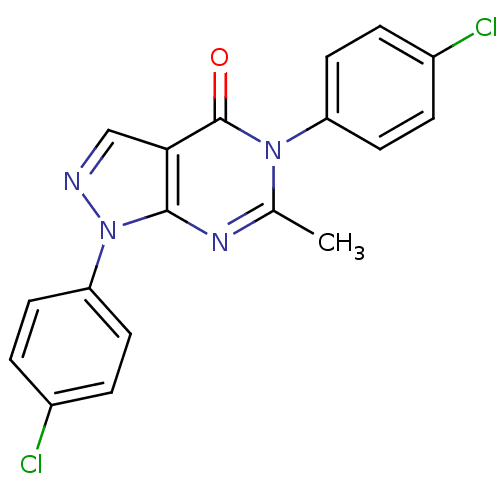 (1,5-bis(4-chlorophenyl)-6-methyl-1H-pyrazolo[3,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50225106 ((2S)-2-amino-5-{[(E)-amino(nitroimino)methyl]amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007922 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214087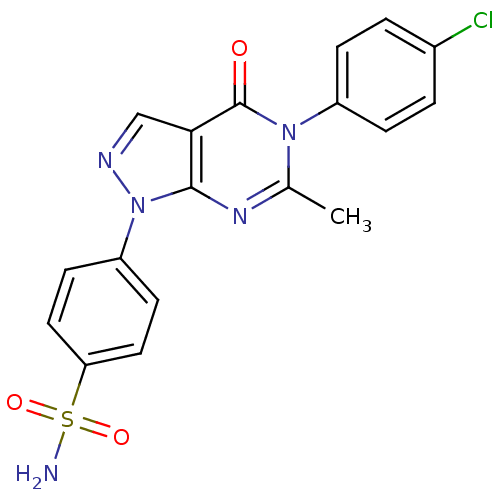 (4-(5-(4-chlorophenyl)-6-methyl-4-oxo-4,5-dihydropy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007925 ((S)-3-[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Cavia porcellus) | BDBM50002477 ((S)-3-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1H...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type A receptor in guinea pig pancreas | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007916 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007926 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007917 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50214090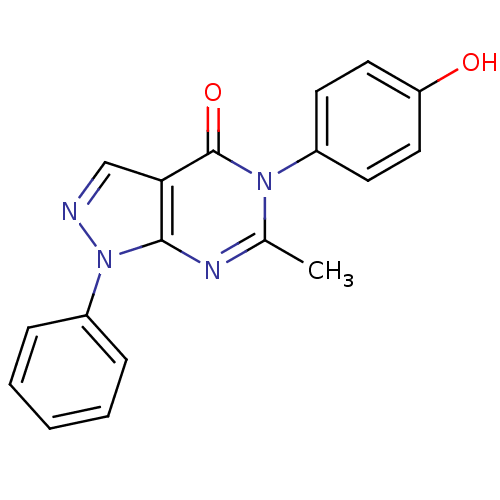 (5-(4-hydroxyphenyl)-6-methyl-1-phenyl-1H-pyrazolo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR1 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065362 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065369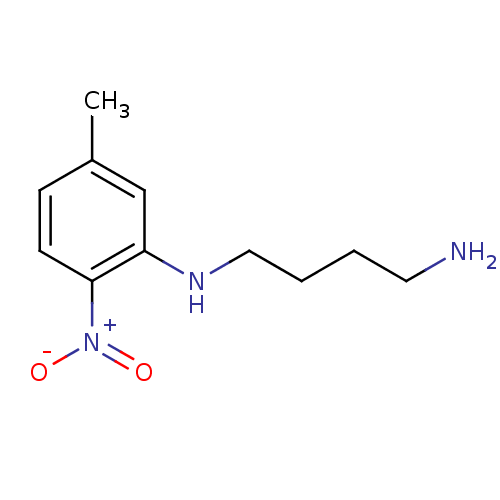 (CHEMBL89454 | N*1*-(5-Methyl-2-nitro-phenyl)-butan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50214076 (6-methyl-1-phenyl-5-(piperidin-1-yl)-1H-pyrazolo[3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR5 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065366 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50214071 (5-(azepan-1-yl)-6-methyl-1-phenyl-1H-pyrazolo[3,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mGluR5 receptor expressed in 132N1 cells assessed as inhibition of glutamate-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 17: 4303-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.028 BindingDB Entry DOI: 10.7270/Q2445M5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007919 (3-{1-[1-aminobutyl oxycarbonylamino-2-(1H-3-indoly...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065364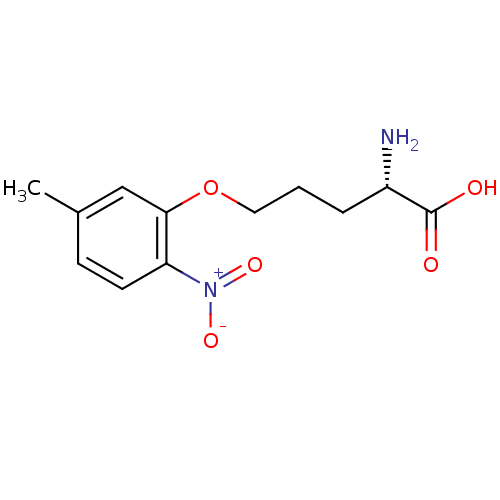 ((S)-2-Amino-5-(5-methyl-2-nitro-phenoxy)-pentanoic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of arginine to citrulline conversion by Neuronal nitric oxide synthase from rat brain | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065359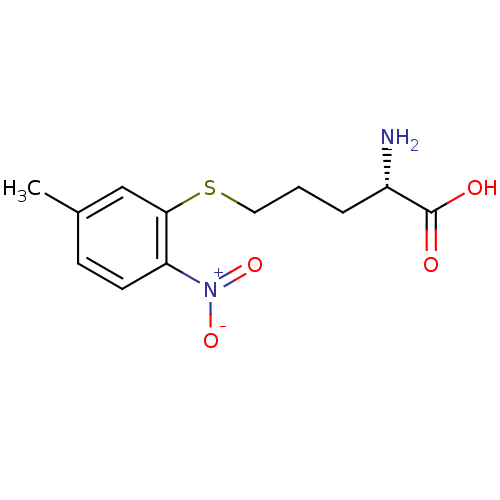 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylsulfanyl)-pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50040523 ((S)-3-{[(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50007923 (3-[5-aminocarboxybenzyl-1-[2-(1H-3-indolyl)-1-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]BH-CCK-8 from Cholecystokinin type B receptor of guinea pig cortex | J Med Chem 33: 2950-2 (1990) BindingDB Entry DOI: 10.7270/Q2M32WCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065362 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of conversion of radiolabeled arginine to citrulline by isomeric form of nitric oxide synthase Endothelial nitric oxide synthase ... | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50065366 ((S)-2-Amino-5-(5-methyl-2-nitro-phenylamino)-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of radiolabeled arginine conversion to citrulline by isomeric form of Inducible nitric oxide synthase from mouse RAW 264.7 cells | J Med Chem 41: 2636-42 (1998) Article DOI: 10.1021/jm980073h BindingDB Entry DOI: 10.7270/Q26Q1WD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 117 total ) | Next | Last >> |