

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046641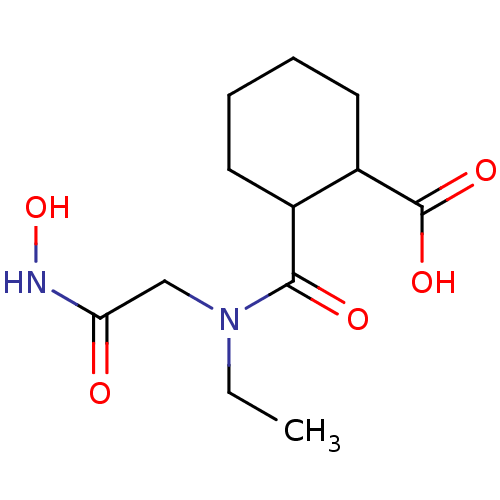 (1R,2R-trans-2-(Ethyl-hydroxycarbamoylmethyl-carbam...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046633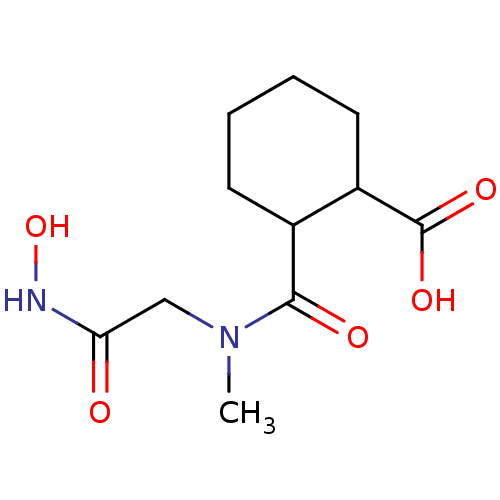 (1R,2R-trans-2-(Hydroxycarbamoylmethyl-methyl-carba...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046633 (1R,2R-trans-2-(Hydroxycarbamoylmethyl-methyl-carba...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046632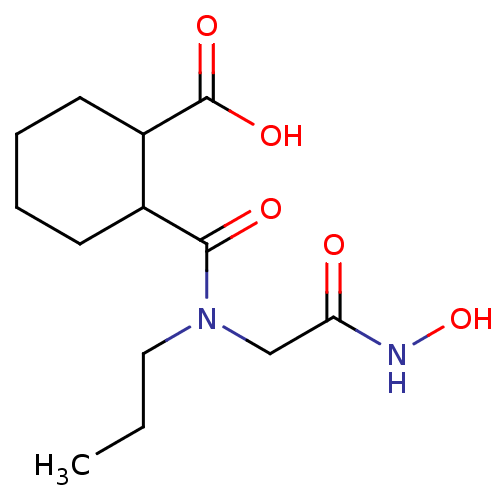 (CHEMBL159829 | Cis-2-(Hydroxycarbamoylmethyl-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046632 (CHEMBL159829 | Cis-2-(Hydroxycarbamoylmethyl-propy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046621 (CHEMBL352453 | Trans-2-(Hydroxycarbamoylmethyl-iso...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046629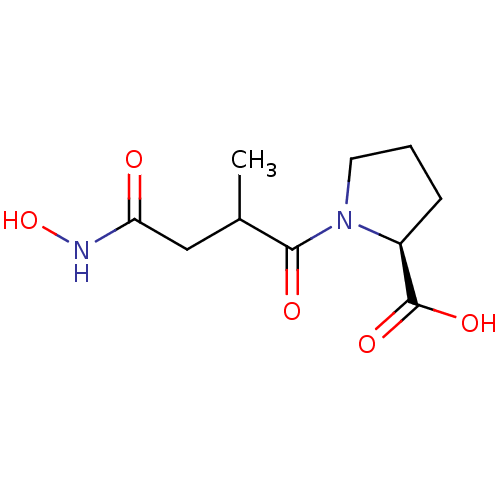 (1-(3-Hydroxycarbamoyl-2-methyl-propionyl)-pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046628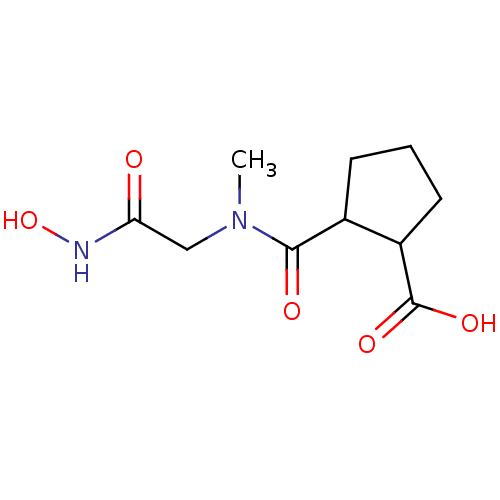 (CHEMBL434293 | Trans-2-(Hydroxycarbamoylmethyl-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046631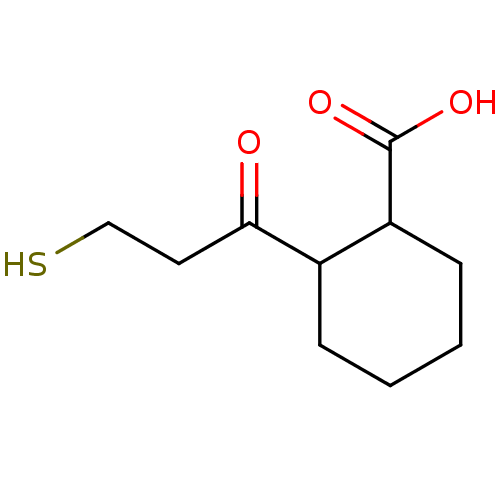 (CHEMBL159791 | Cis-2-(3-Mercapto-propionyl)-cycloh...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046631 (CHEMBL159791 | Cis-2-(3-Mercapto-propionyl)-cycloh...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046641 (1R,2R-trans-2-(Ethyl-hydroxycarbamoylmethyl-carbam...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046634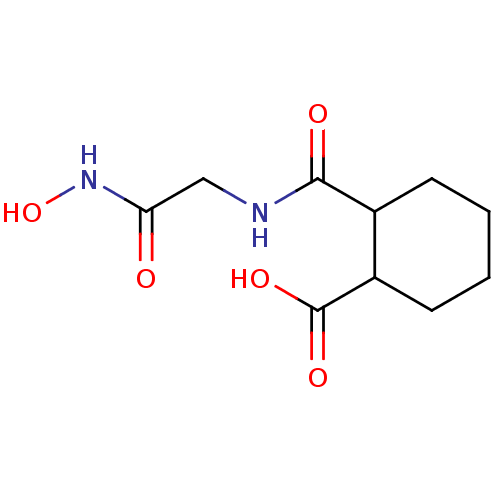 (CHEMBL161968 | Cis-2-(Hydroxycarbamoylmethyl-carba...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046634 (CHEMBL161968 | Cis-2-(Hydroxycarbamoylmethyl-carba...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046638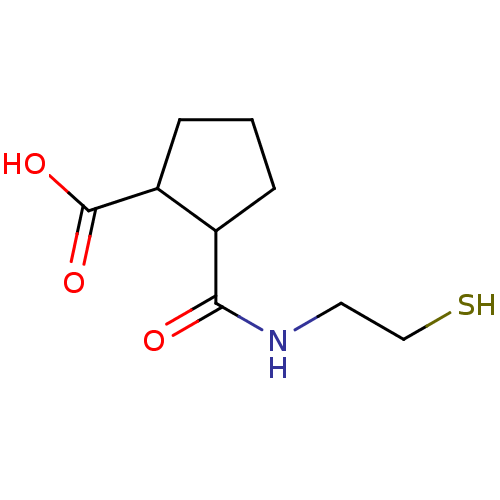 (CHEMBL163601 | Cis-2-(2-Mercapto-ethylcarbamoyl)-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046630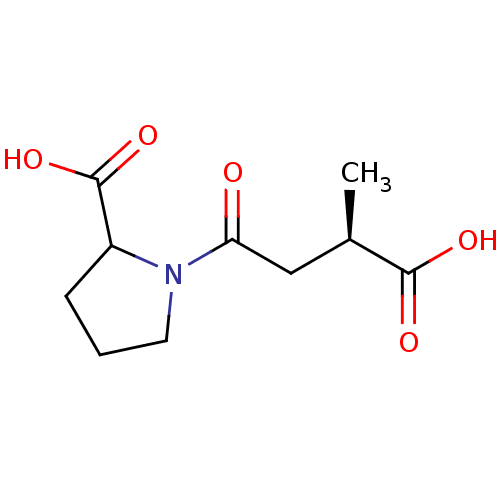 (1-(3-Carboxy-butyryl)-pyrrolidine-2-carboxylic aci...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046626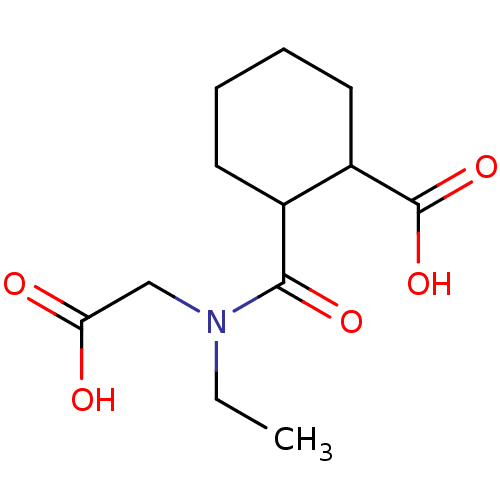 (CHEMBL163568 | Cis-2-(Carboxymethyl-ethyl-carbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046626 (CHEMBL163568 | Cis-2-(Carboxymethyl-ethyl-carbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046642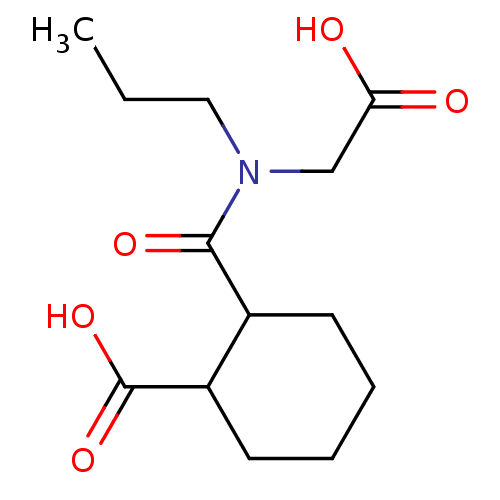 (CHEMBL159987 | Cis-2-(Carboxymethyl-propyl-carbamo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046639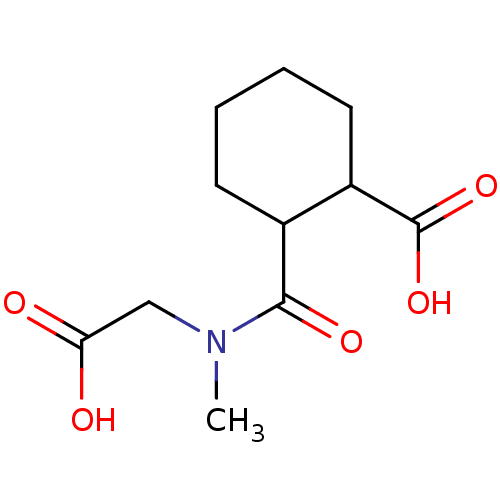 (CHEMBL159330 | Cis-2-(Carboxymethyl-methyl-carbamo...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046637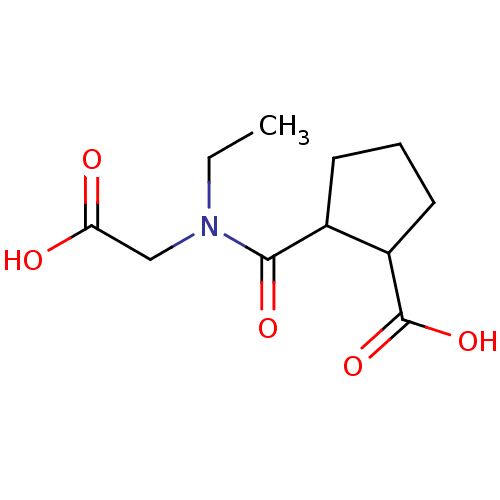 (CHEMBL351263 | Cis-2-(Carboxymethyl-ethyl-carbamoy...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046635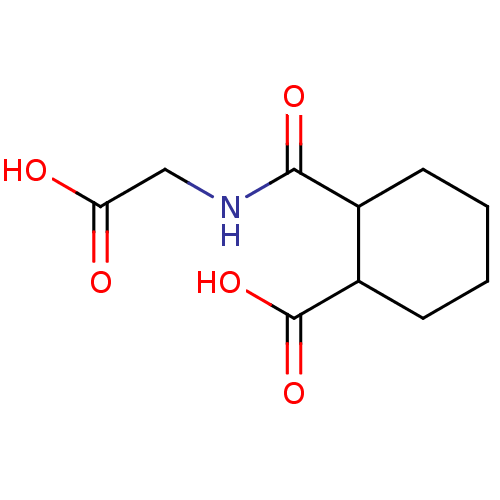 (CHEMBL159973 | Cis-2-(Carboxymethyl-carbamoyl)-cyc...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046622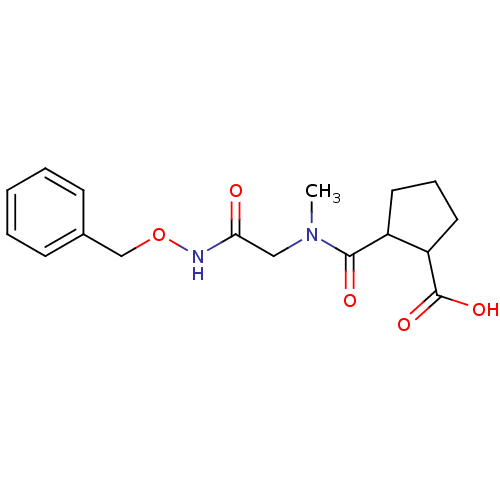 (CHEMBL159995 | Trans-2-[(Benzyloxycarbamoyl-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046625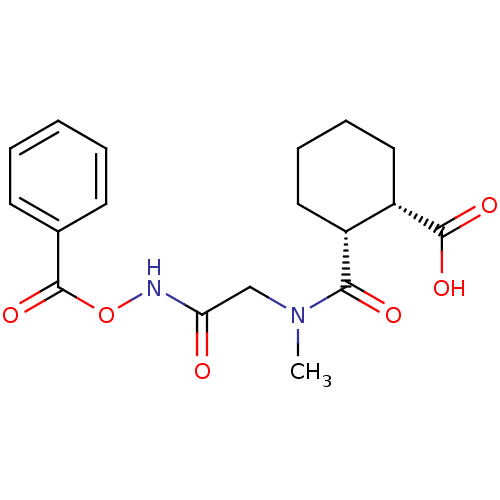 (1R,2R-trans-2-[(Benzoyloxycarbamoyl-methyl)-carbam...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046620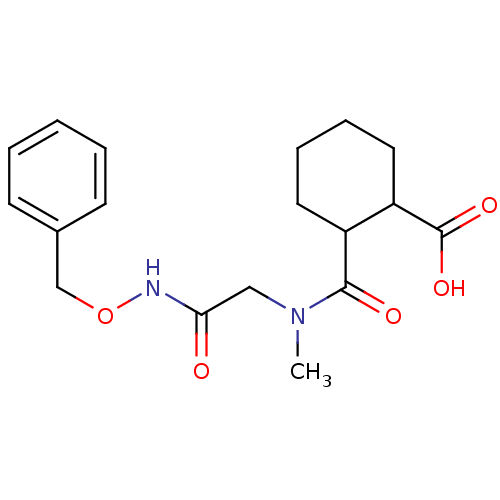 (CHEMBL159956 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046624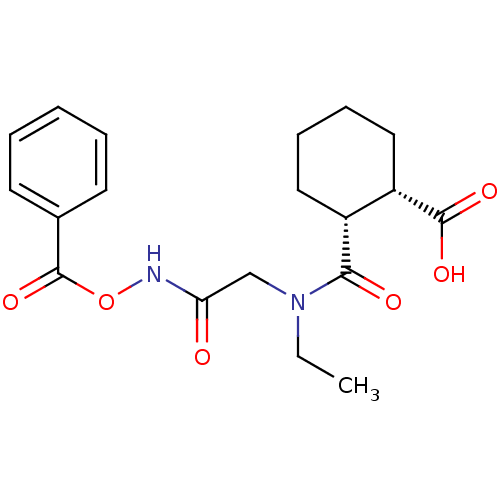 (1R,2R-trans-2-[(Benzoyloxycarbamoyl-methyl)-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046627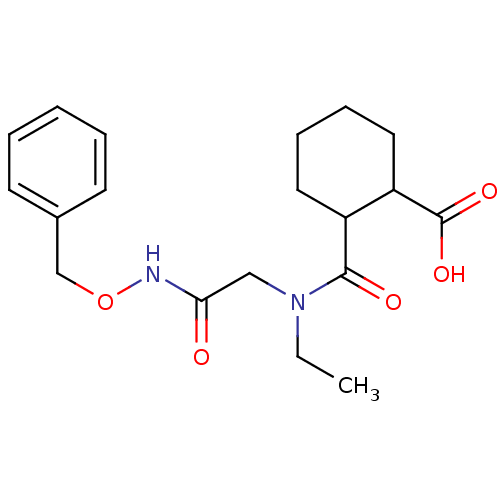 (CHEMBL161552 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046640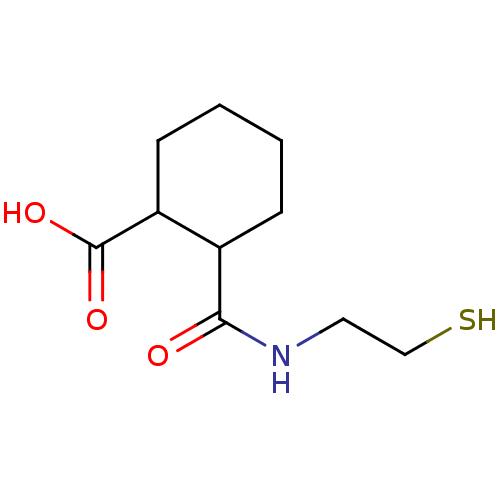 (CHEMBL163549 | Cis-2-(2-Mercapto-ethylcarbamoyl)-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046636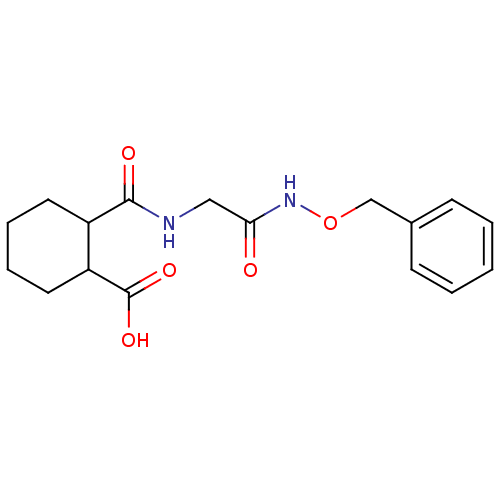 (CHEMBL159996 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046627 (CHEMBL161552 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046640 (CHEMBL163549 | Cis-2-(2-Mercapto-ethylcarbamoyl)-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046625 (1R,2R-trans-2-[(Benzoyloxycarbamoyl-methyl)-carbam...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046636 (CHEMBL159996 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046620 (CHEMBL159956 | Cis-2-[(Benzyloxycarbamoyl-methyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046624 (1R,2R-trans-2-[(Benzoyloxycarbamoyl-methyl)-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50046623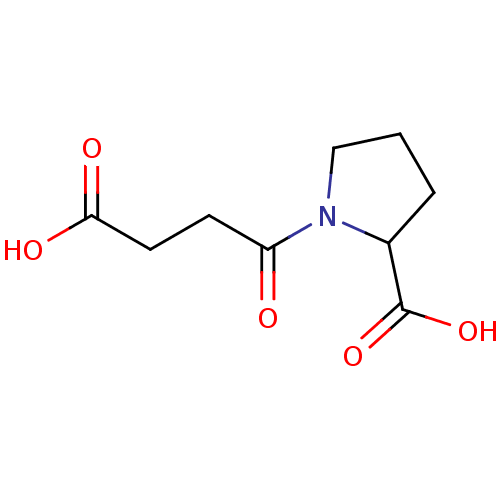 (1-(3-Carboxy-propionyl)-pyrrolidine-2-carboxylic a...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori Guidotti S.p.A. Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat serum angiotensin I converting enzyme using hippuryl-glycyl-glycine as substrate | J Med Chem 36: 699-707 (1993) BindingDB Entry DOI: 10.7270/Q21N81RS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||