

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Toxoplasma gondii) | BDBM50200328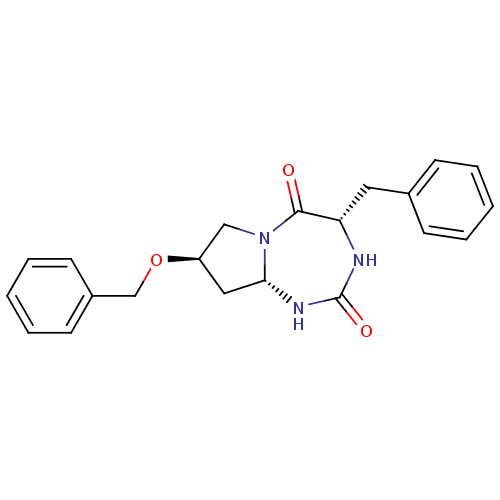 ((4S,8R,9aS)-4-benzyl-8-(benzyloxy)-hexahydro-1H-py...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii purine nucleoside phosphorylase | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor receptor superfamily member 5 (Homo sapiens (Human)) | BDBM50325995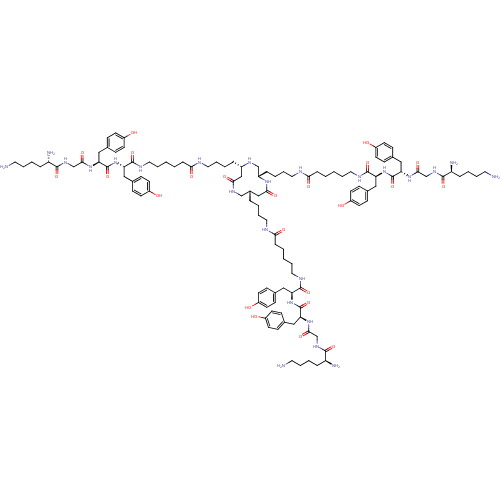 ((2S,2'S,2''S)-N,N',N''-((4S,4'S,4''S,7S,7'S,7''S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Biologie Moléculaire et Cellulaire Curated by ChEMBL | Assay Description Inhibition of recombinant human CD40L:mouse CD8 tail binding to human CD40 by surface plasmon resonance method | Nat Chem Biol 1: 377-82 (2005) BindingDB Entry DOI: 10.7270/Q2WD40SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor receptor superfamily member 5 (Homo sapiens (Human)) | BDBM50325994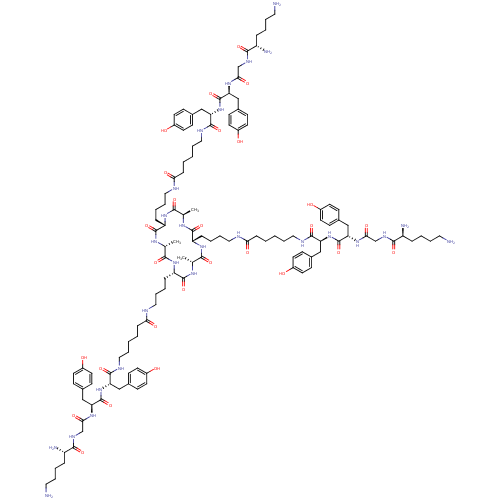 ((2S,2'S,2''S)-N,N',N''-((4S,4'S,4''S,7S,7'S,7''S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Biologie Moléculaire et Cellulaire Curated by ChEMBL | Assay Description Inhibition of recombinant human CD40L:mouse CD8 tail binding to human CD40 by surface plasmon resonance method | Nat Chem Biol 1: 377-82 (2005) BindingDB Entry DOI: 10.7270/Q2WD40SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tumor necrosis factor receptor superfamily member 5 (Homo sapiens (Human)) | BDBM50325996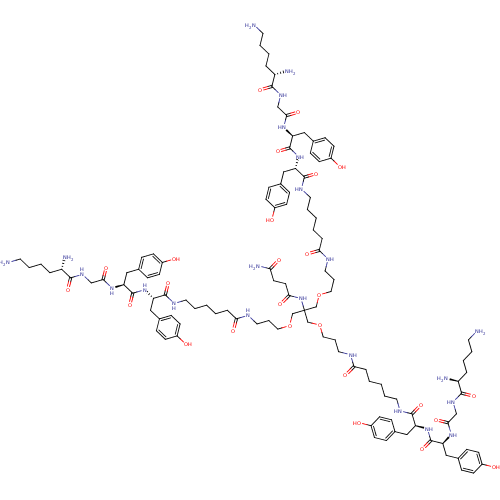 (CHEMBL1241019 | N1-((5S,11S,14S,44S,47S,53S)-1,5,5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Biologie Moléculaire et Cellulaire Curated by ChEMBL | Assay Description Inhibition of recombinant human CD40L:mouse CD8 tail binding to human CD40 by surface plasmon resonance method | Nat Chem Biol 1: 377-82 (2005) BindingDB Entry DOI: 10.7270/Q2WD40SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50200327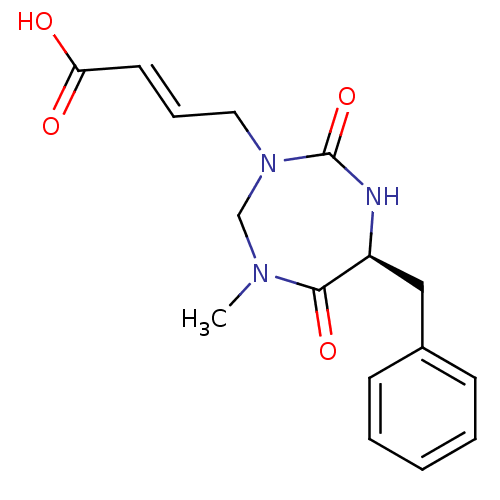 ((E)-4-(6-Benzyl-1-methyl-4,7-dioxo-[1,3,5]triazepa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of human group 10 sPLA2 | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50200326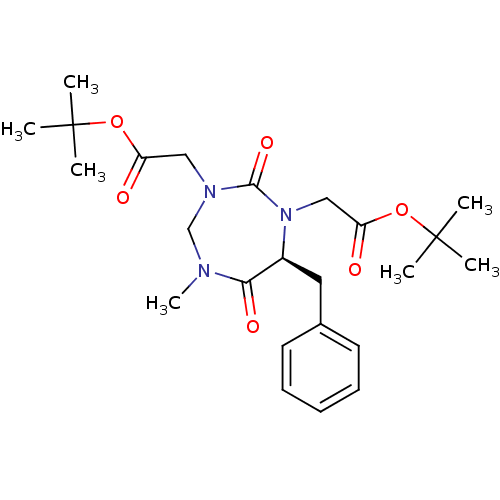 (((S)-7-benzyl-3-tert-butoxycarbonylmethyl-5-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of human group 10 sPLA2 | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50200326 (((S)-7-benzyl-3-tert-butoxycarbonylmethyl-5-methyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of human group 5 sPLA2 | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50200325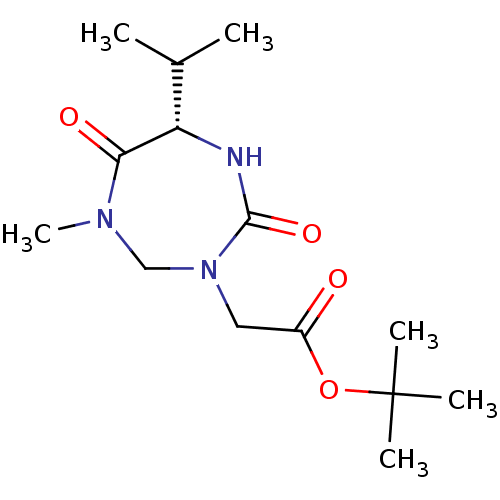 ((S)-tert-butyl 2-(6-isopropyl-1-methyl-4,7-dioxo-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of human group 10 sPLA2 | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50200324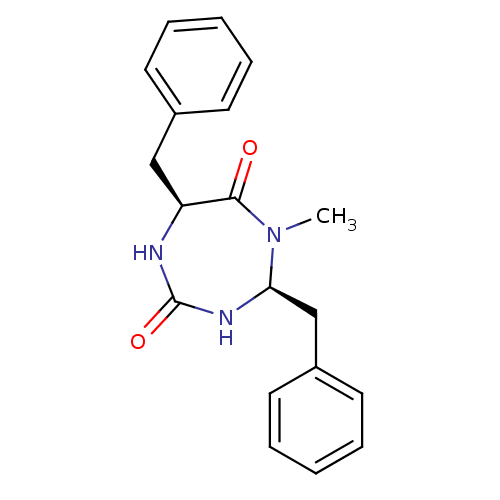 ((4S,7S)-4,7-dibenzyl-5-methyl-1,3,5-triazepane-2,6...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of human group 5 sPLA2 | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50200325 ((S)-tert-butyl 2-(6-isopropyl-1-methyl-4,7-dioxo-1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of human group 5 sPLA2 | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50200327 ((E)-4-(6-Benzyl-1-methyl-4,7-dioxo-[1,3,5]triazepa...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of human group 5 sPLA2 | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50200324 ((4S,7S)-4,7-dibenzyl-5-methyl-1,3,5-triazepane-2,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS UMR 7175 Curated by ChEMBL | Assay Description Inhibition of human group 10 sPLA2 | J Med Chem 49: 6768-78 (2006) Article DOI: 10.1021/jm0606589 BindingDB Entry DOI: 10.7270/Q2WH2PND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||