

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50254908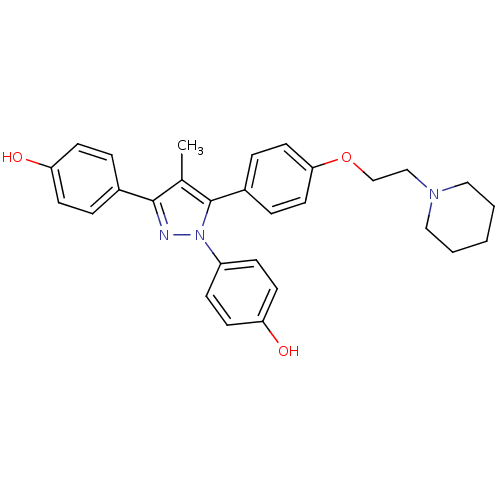 (4-[1-(4-hydroxyphenyl)-4-methyl-5-{4-[2-(piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of ERalpha in human MCF7 cells | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50528756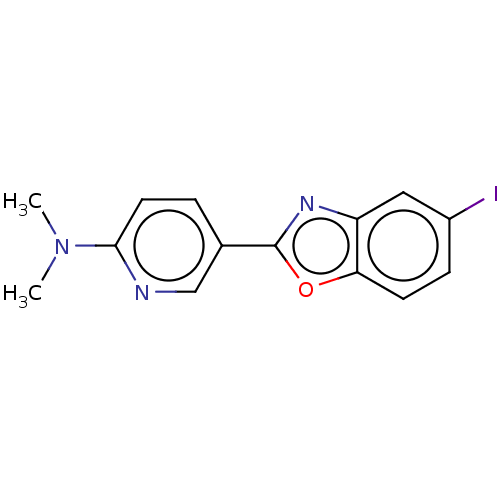 (CHEMBL4470128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Displacement of [125I]IMPY from human amyloid beta(1-42) aggregates incubated for 3 hrs by gamma counting analysis | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111613 BindingDB Entry DOI: 10.7270/Q2GQ726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50458333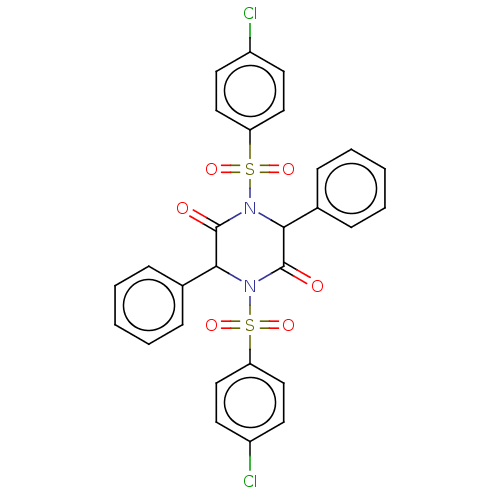 (CHEMBL4210820) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition b... | Eur J Med Chem 150: 87-101 (2018) Article DOI: 10.1016/j.ejmech.2018.02.078 BindingDB Entry DOI: 10.7270/Q2SJ1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50458333 (CHEMBL4210820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Competitive inhibition of human MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH as substrate preincubated for 60 mins followed by substrate addition by... | Eur J Med Chem 150: 87-101 (2018) Article DOI: 10.1016/j.ejmech.2018.02.078 BindingDB Entry DOI: 10.7270/Q2SJ1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50528740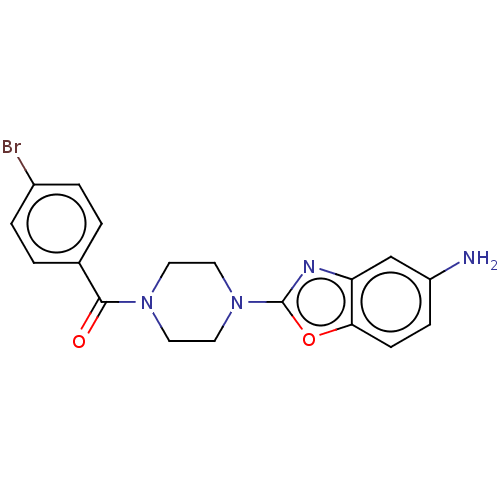 (CHEMBL4536953) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using varying levels of acetylthiocholine iodide as substrate measured at 2 mins interval for 10 mins... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111613 BindingDB Entry DOI: 10.7270/Q2GQ726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50525255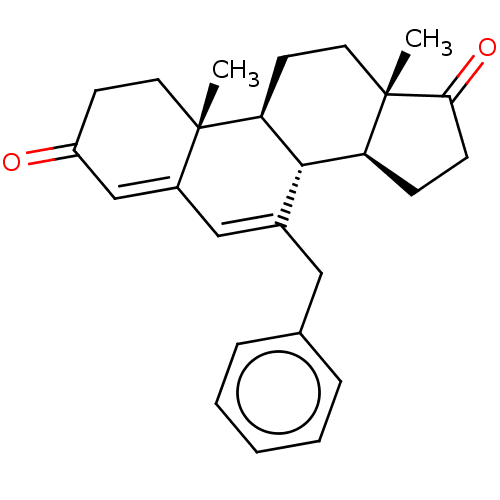 (CHEMBL3754505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50525268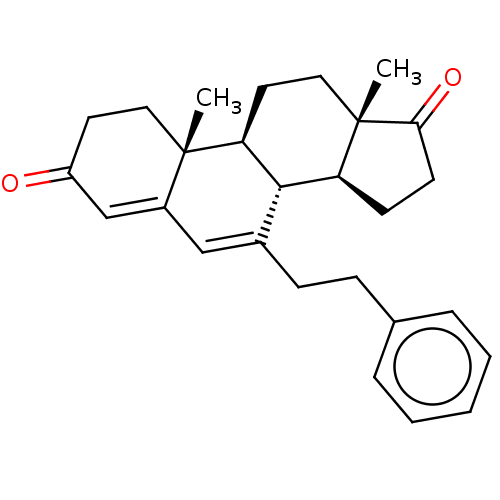 (CHEMBL3753089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9454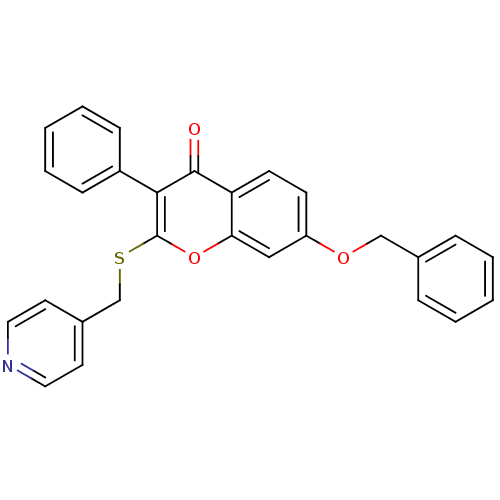 (7-(benzyloxy)-3-phenyl-2-[(pyridin-4-ylmethyl)sulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta[3H]]androst-4-ene-3,17-dione as substrate after 15 mins in presence of NADPH by liquid... | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50525262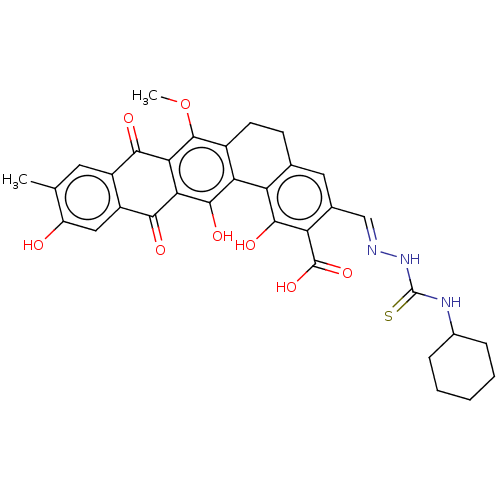 (CHEMBL4444347) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) using [3H]E1S as substrate after 20 mins by scintillation counting method | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528740 (CHEMBL4536953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum BuChE using varying levels of butyrylthiocholine iodide as substrate measured at 2 mins interval for 10 mi... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111613 BindingDB Entry DOI: 10.7270/Q2GQ726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50254908 (4-[1-(4-hydroxyphenyl)-4-methyl-5-{4-[2-(piperidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of ERbeta in human MCF7 cells | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9461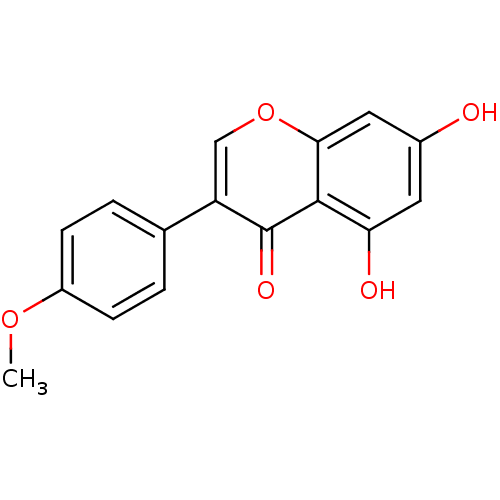 (5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate after 15 mins in presence of NADPH by liquid scin... | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50370691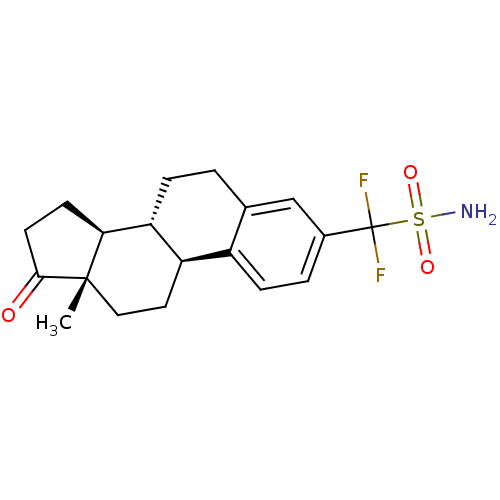 (CHEMBL1628004) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50073853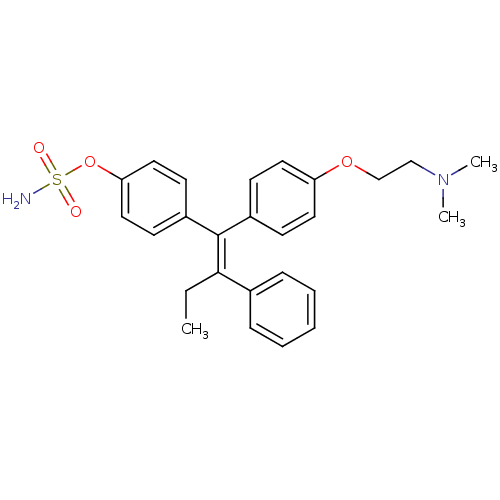 (CHEMBL33862 | Sulfamic acid 4-{(E)-1-[4-(2-dimethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50073852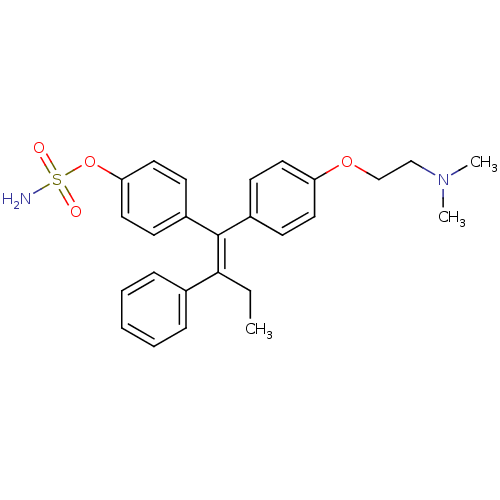 (CHEMBL32172 | Sulfamic acid 4-{(Z)-1-[4-(2-dimethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50525253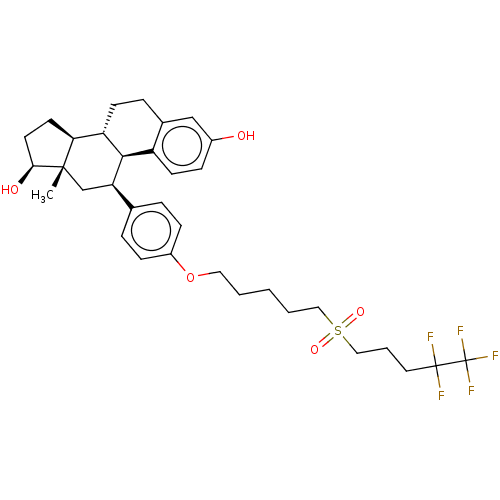 (CHEMBL4518283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of ERalpha in human MCF7 cells after 36 hrs by Western blot analysis | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50118551 (CHEMBL137392 | Sulfamic acid 2-tert-butyl-4-oxo-4H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of human sulfatase using 4-methylumbelliferyl sulfate as substrate after 60 mins | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50118567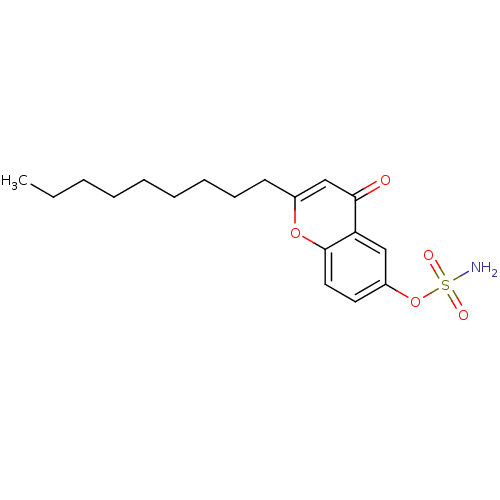 (CHEMBL137692 | Sulfamic acid 2-nonyl-4-oxo-4H-chro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of human sulfatase using 4-methylumbelliferyl sulfate as substrate after 60 mins | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50525257 (CHEMBL4520995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate by liquid scintillation counting method | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369432 (CHEMBL1627465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) using [3H]E1S as substrate after 18 hrs | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50525260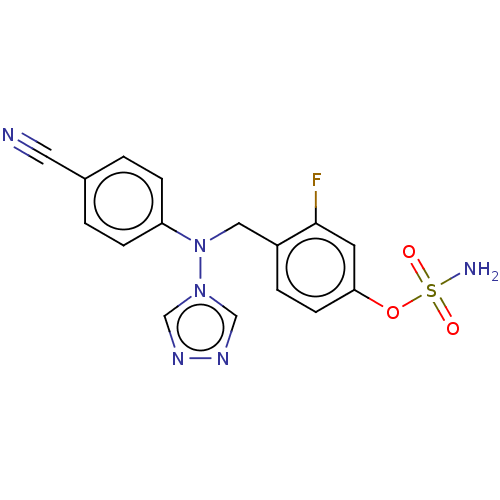 (CHEMBL4465348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50121079 (CHEMBL3622064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50121105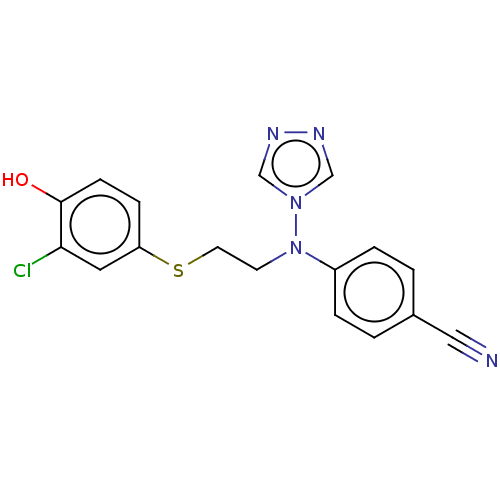 (CHEMBL3622055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50118560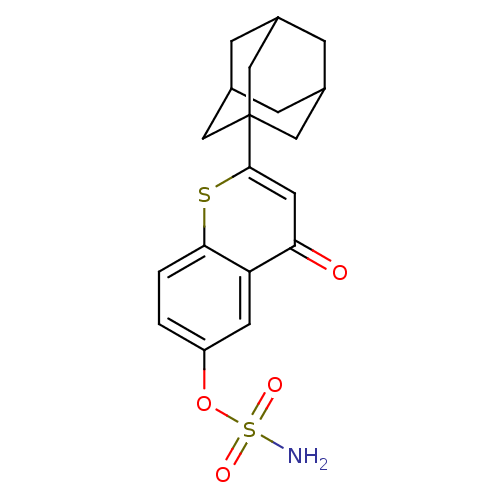 (CHEMBL262050 | Sulfamic acid 2-adamantan-1-yl-4-ox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) using 4-MUS as substrate | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50291665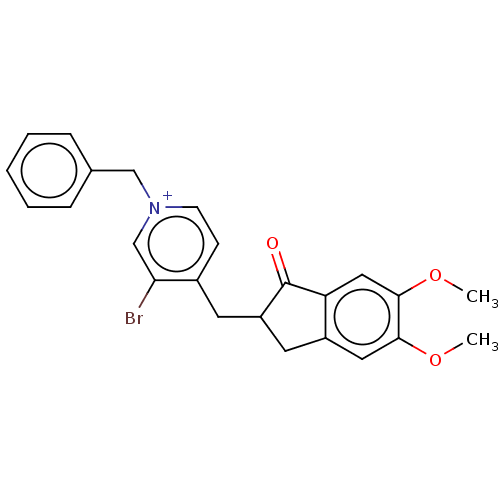 (CHEMBL4165327) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and m... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111613 BindingDB Entry DOI: 10.7270/Q2GQ726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369431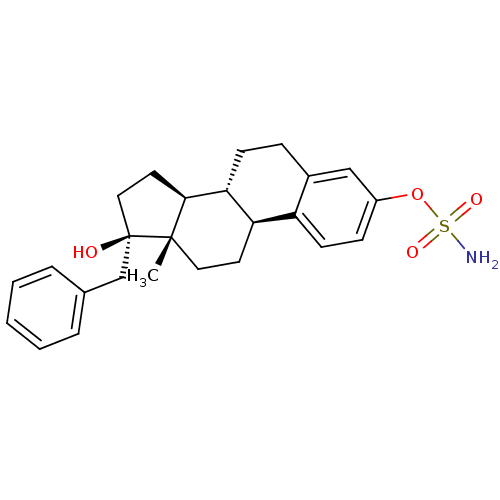 (CHEMBL1627878) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) using [3H]E1S as substrate after 18 hrs | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10016 (4-{[(4-bromophenyl)methyl](4H-1,2,4-triazol-4-yl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of human placental microsome aromatase using [1beta,2beta3H]androstenedione as substrate by liquid scintillation counting method | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50047259 (4-(1-(1H-imidazol-1-yl)vinyl)benzonitrile | 4-(1-I...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10020 ((2-bromo-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369432 (CHEMBL1627465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) using [3H]DHEAS as substrate after 18 hrs | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50121078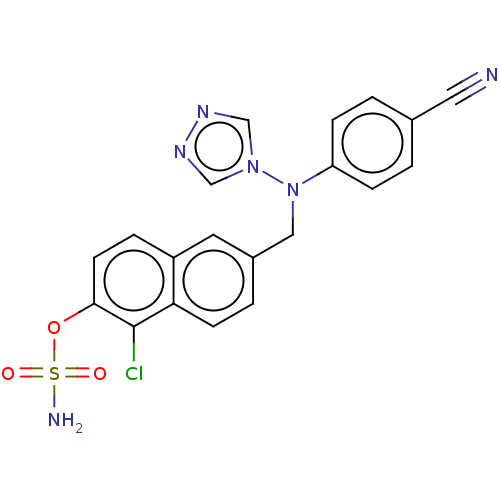 (CHEMBL3622063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM10019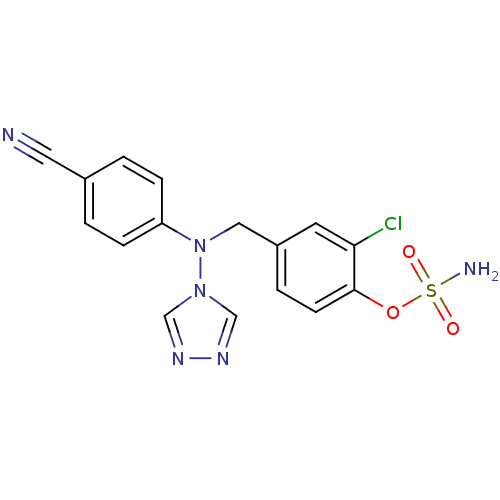 ((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of aromatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50525260 (CHEMBL4465348) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50041611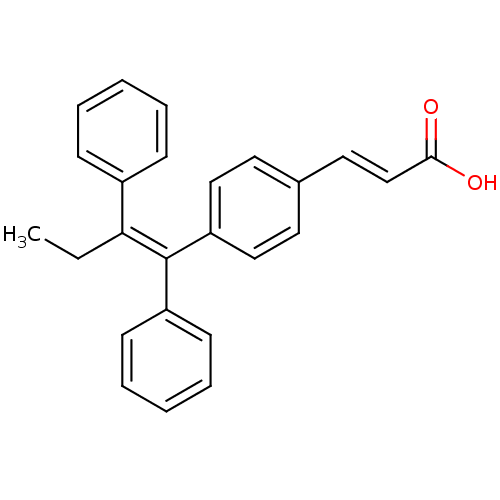 ((2E)-3-{4-[(1E)-1,2-DIPHENYLBUT-1-ENYL]PHENYL}ACRY...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of Fluormone-ES2 binding affinity to ERalpha (unknown origin) after 2 hrs by fluorescent polarization based competition binding assay | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50121196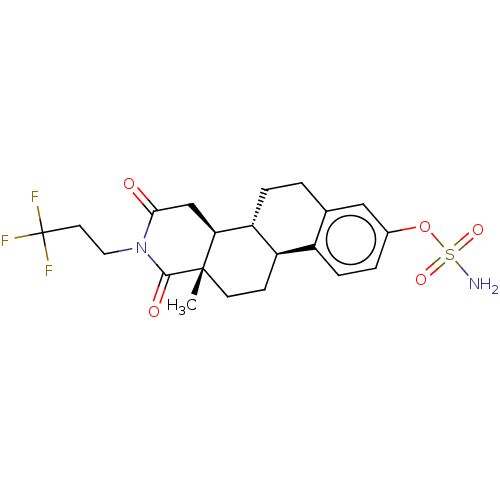 (CHEMBL3622029) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50525263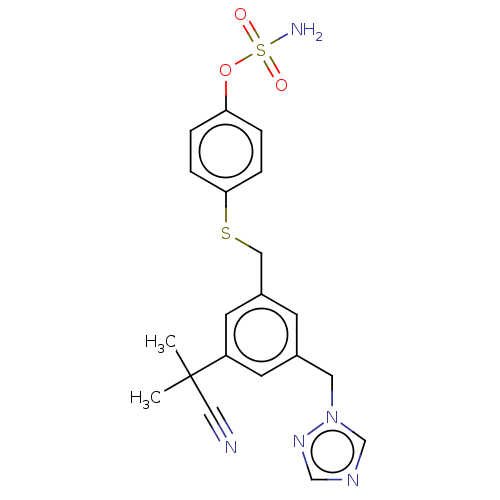 (CHEMBL4444074) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of ERalpha (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50369431 (CHEMBL1627878) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) using [3H]DHEAS as substrate after 18 hrs | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50525261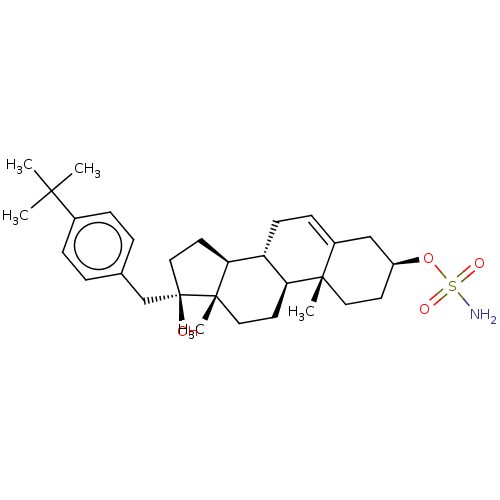 (CHEMBL4436482) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) expressed in HEK293 cells using [3H]DHEAS as substrate after 2 hrs by liquid scintillation counting method | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM13080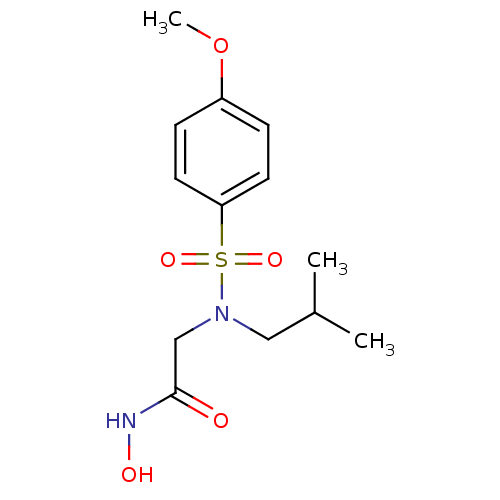 (CGS 27023A Analog 3 | N-hydroxy-2-[(4-methoxybenze...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Inhibition of human MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH as substrate preincubated for 60 mins followed by substrate addition by fluorescenc... | Eur J Med Chem 150: 87-101 (2018) Article DOI: 10.1016/j.ejmech.2018.02.078 BindingDB Entry DOI: 10.7270/Q2SJ1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50458333 (CHEMBL4210820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Inhibition of human MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH as substrate preincubated for 60 mins followed by substrate addition by fluorescenc... | Eur J Med Chem 150: 87-101 (2018) Article DOI: 10.1016/j.ejmech.2018.02.078 BindingDB Entry DOI: 10.7270/Q2SJ1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM10019 ((2-chloro-4-{[(4-cyanophenyl)(4H-1,2,4-triazol-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition and measured for ... | Eur J Med Chem 150: 87-101 (2018) Article DOI: 10.1016/j.ejmech.2018.02.078 BindingDB Entry DOI: 10.7270/Q2SJ1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and m... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111613 BindingDB Entry DOI: 10.7270/Q2GQ726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50363594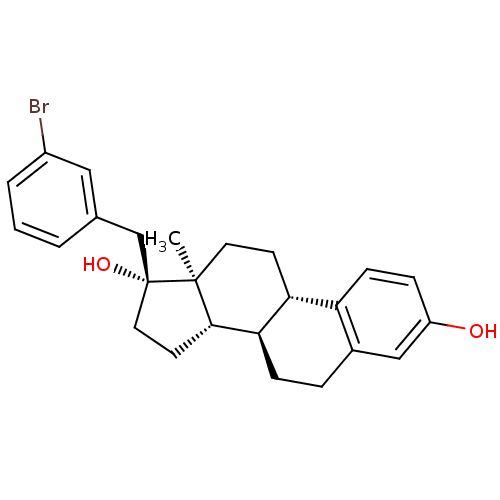 (CHEMBL1627429) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50121078 (CHEMBL3622063) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50366532 (CHEMBL518966) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of sulfatase (unknown origin) | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50197875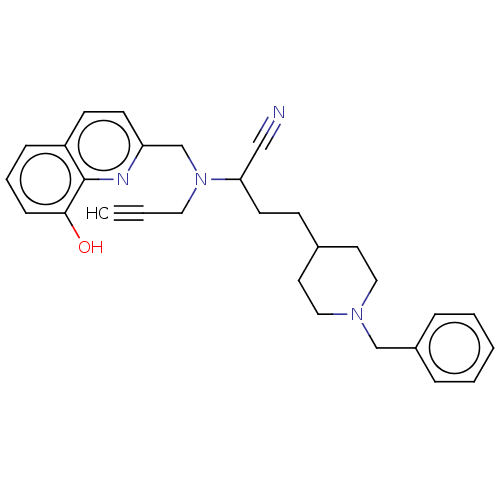 (CHEMBL3910142) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 30 mins followed by substrate addition and m... | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111613 BindingDB Entry DOI: 10.7270/Q2GQ726C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50458333 (CHEMBL4210820) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (Banaras Hindu University) Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 30 mins followed by substrate addition and measured for ... | Eur J Med Chem 150: 87-101 (2018) Article DOI: 10.1016/j.ejmech.2018.02.078 BindingDB Entry DOI: 10.7270/Q2SJ1P72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steryl-sulfatase (Homo sapiens (Human)) | BDBM50121058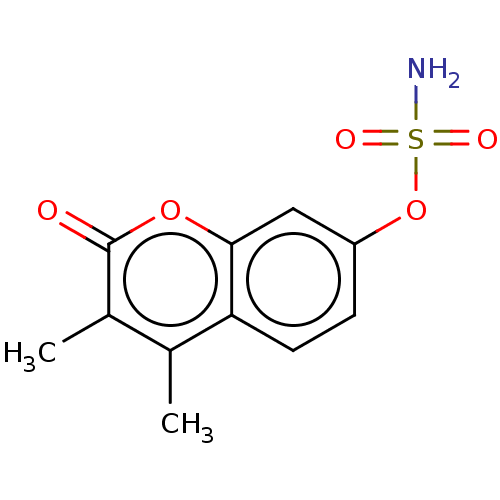 (CHEMBL275331) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of human sulfatase using 4-methylumbelliferyl sulfate as substrate after 60 mins | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50525266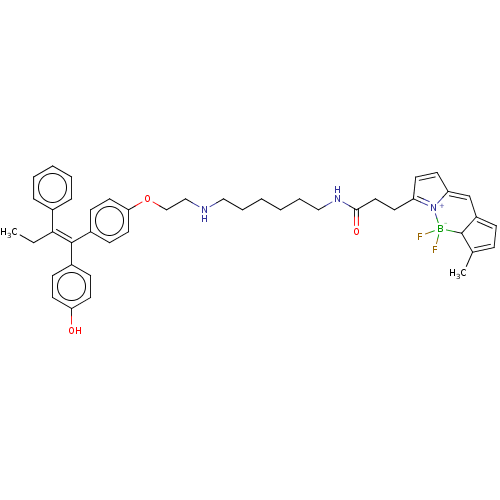 (CHEMBL4547986) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU) Curated by ChEMBL | Assay Description Inhibition of ERalpha (unknown origin) after 18 hrs by dual luciferase reporter gene assay | Eur J Med Chem 177: 116-143 (2019) Article DOI: 10.1016/j.ejmech.2019.05.023 BindingDB Entry DOI: 10.7270/Q2W099B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 221 total ) | Next | Last >> |