

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021311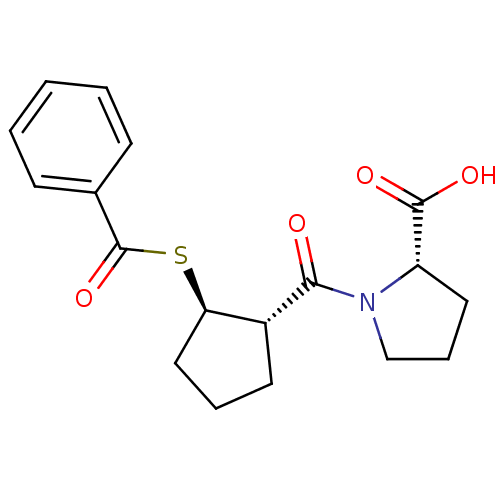 (1-(2-Benzoylsulfanyl-cyclopentanecarbonyl)-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021303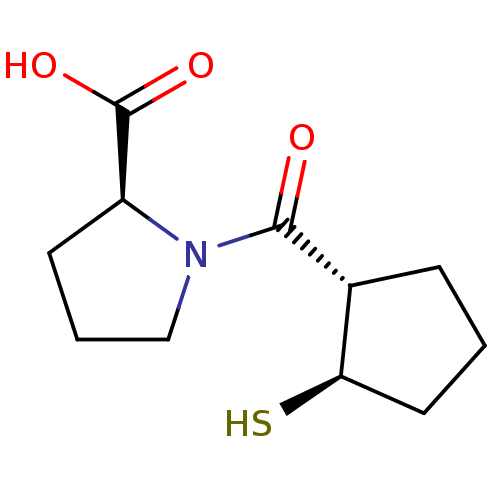 (1-(2-Mercapto-cyclopentanecarbonyl)-pyrrolidine-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021312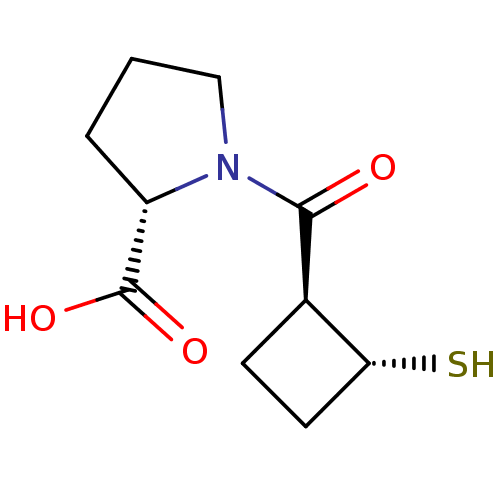 (1-(2-Mercapto-cyclobutanecarbonyl)-pyrrolidine-2-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021308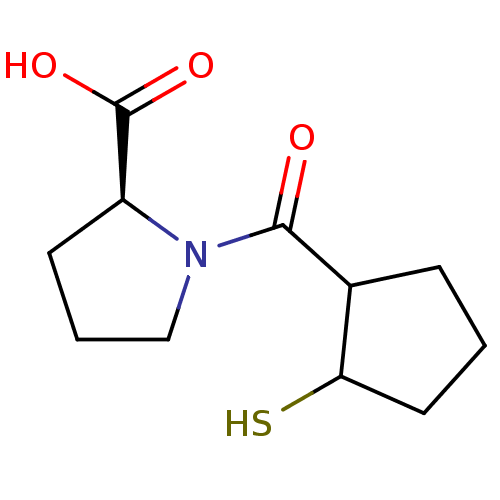 (1-(2-Mercapto-cyclopentanecarbonyl)-pyrrolidine-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021306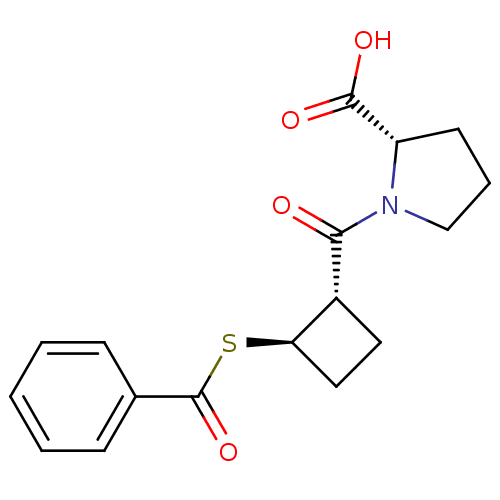 (1-(2-Benzoylsulfanyl-cyclobutanecarbonyl)-pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021317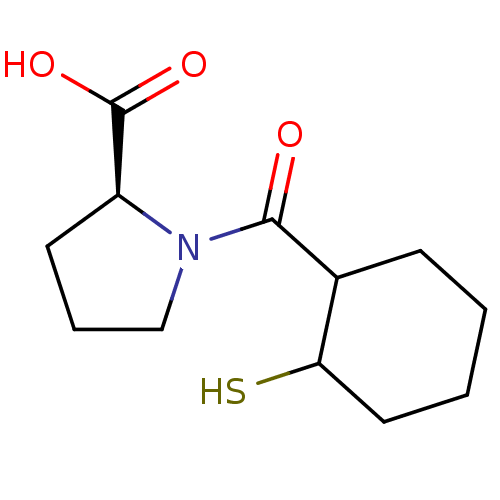 (1-(2-Mercapto-cyclohexanecarbonyl)-pyrrolidine-2-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021314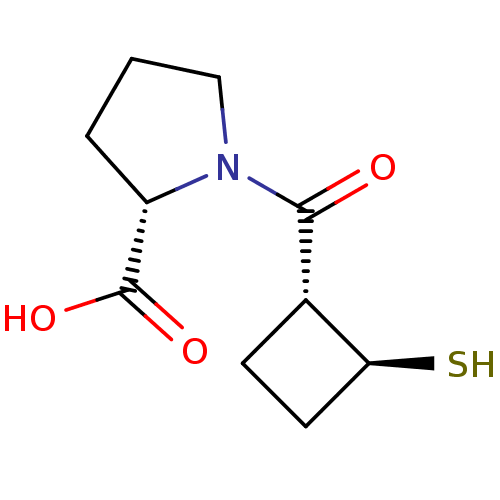 (1-(2-Mercapto-cyclobutanecarbonyl)-pyrrolidine-2-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021297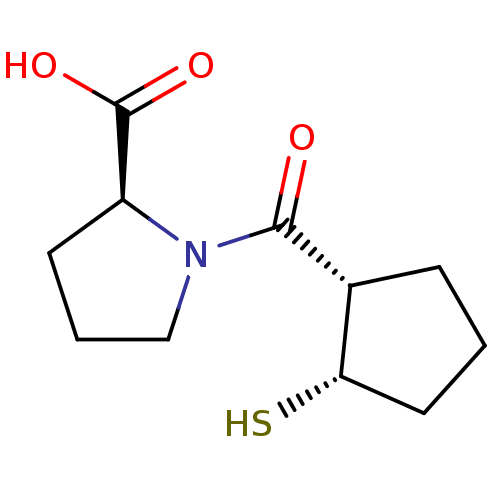 (1-(2-Mercapto-cyclopentanecarbonyl)-pyrrolidine-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021319 (1-(2-Mercapto-cyclopentanecarbonyl)-pyrrolidine-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021299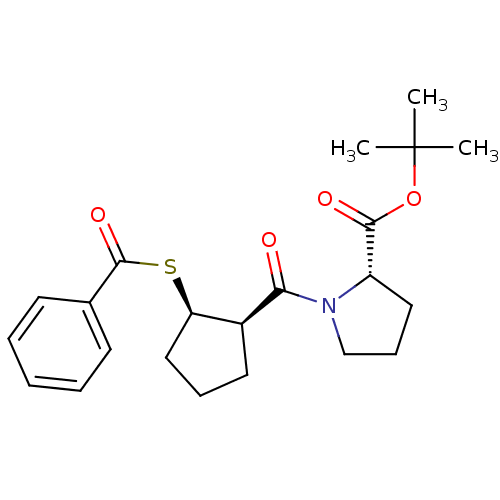 (1-(2-Benzoylsulfanyl-cyclopentanecarbonyl)-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021307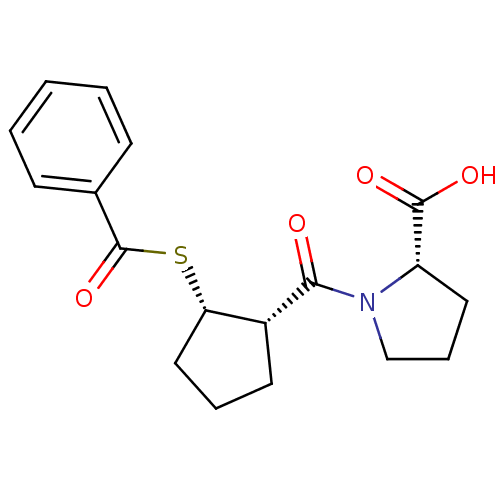 (1-(2-Benzoylsulfanyl-cyclopentanecarbonyl)-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021305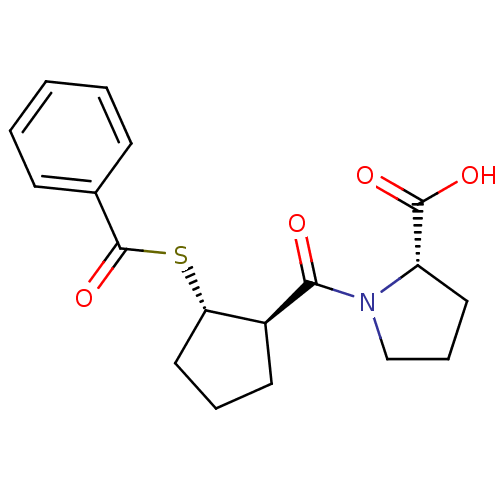 (1-(2-Benzoylsulfanyl-cyclopentanecarbonyl)-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021301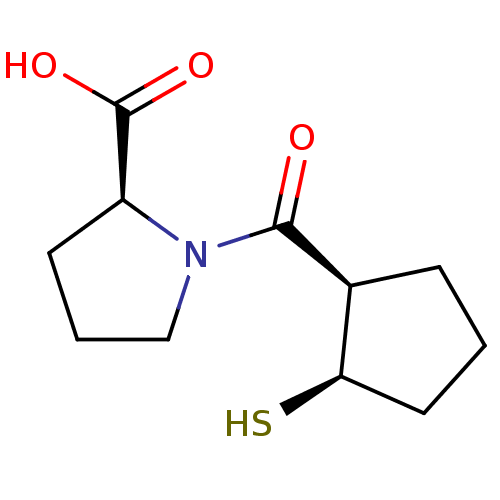 (1-(2-Mercapto-cyclopentanecarbonyl)-pyrrolidine-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021310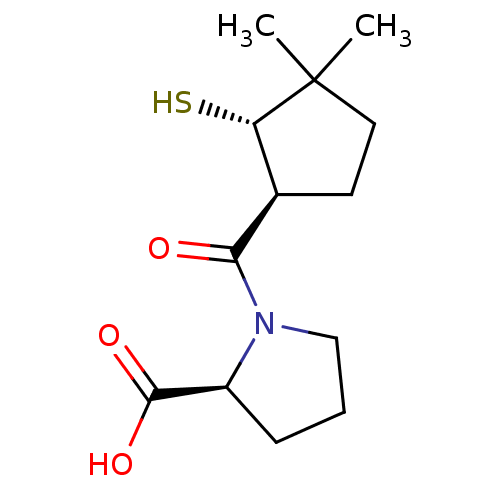 (1-(2-Mercapto-3,3-dimethyl-cyclopentanecarbonyl)-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021309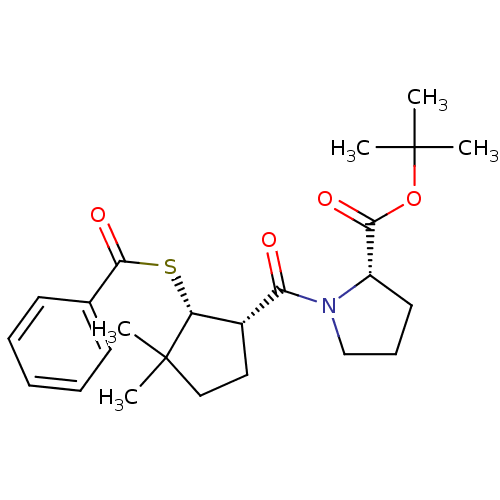 (1-(2-Benzoylsulfanyl-3,3-dimethyl-cyclopentanecarb...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021304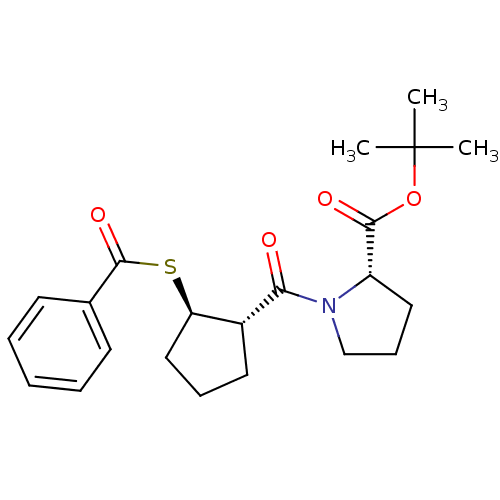 (1-(2-Benzoylsulfanyl-cyclopentanecarbonyl)-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021313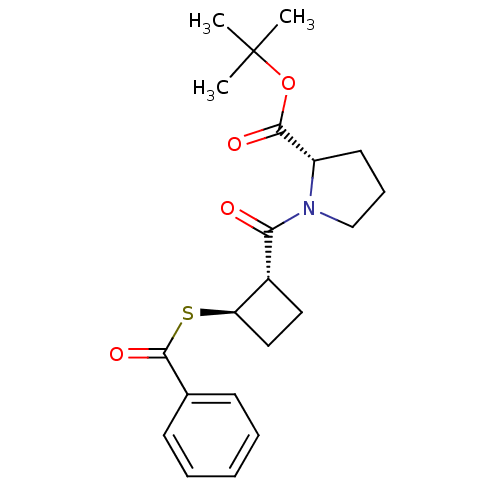 (1-(2-Benzoylsulfanyl-cyclobutanecarbonyl)-pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021302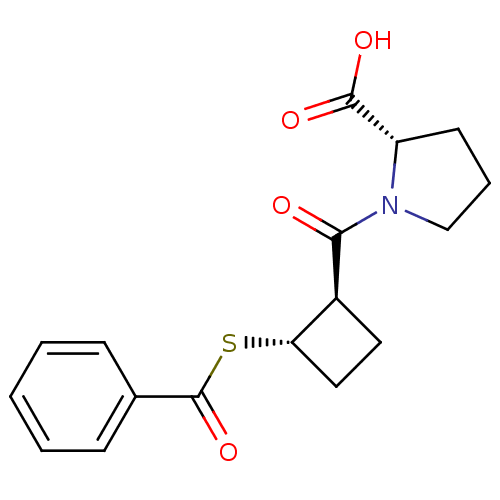 (1-(2-Benzoylsulfanyl-cyclobutanecarbonyl)-pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021315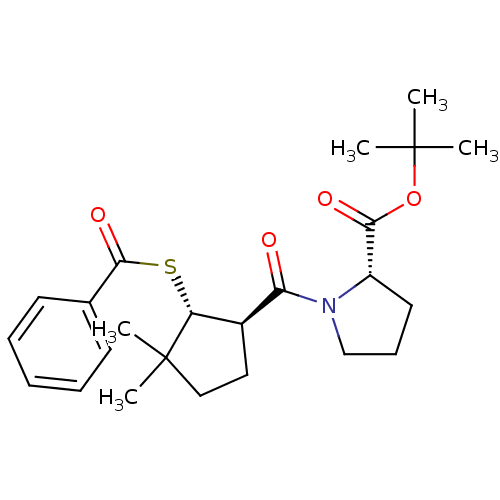 (1-(2-Benzoylsulfanyl-3,3-dimethyl-cyclopentanecarb...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021321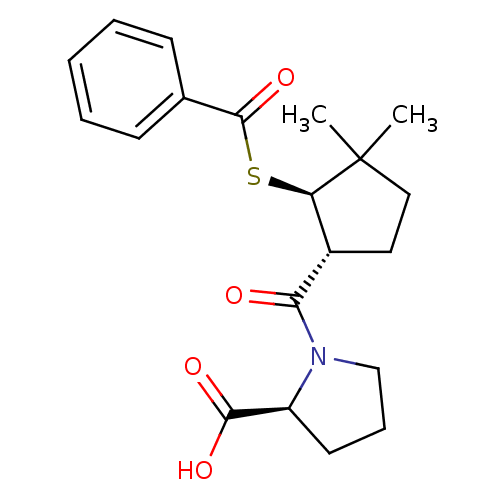 (1-(2-Benzoylsulfanyl-3,3-dimethyl-cyclopentanecarb...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021298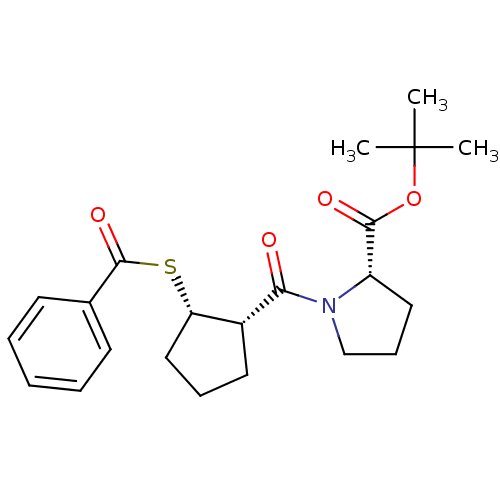 (1-(2-Benzoylsulfanyl-cyclopentanecarbonyl)-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021316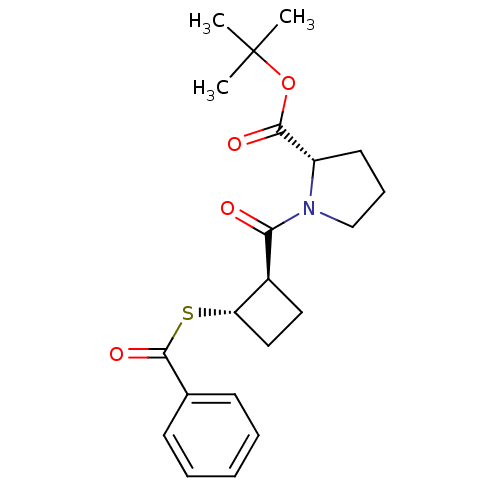 (1-(2-Benzoylsulfanyl-cyclobutanecarbonyl)-pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021318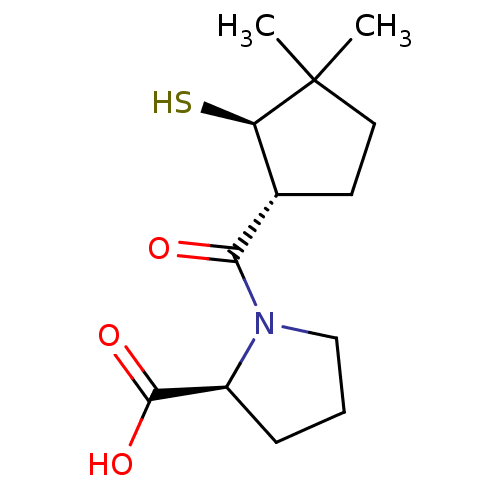 (1-(2-Mercapto-3,3-dimethyl-cyclopentanecarbonyl)-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021320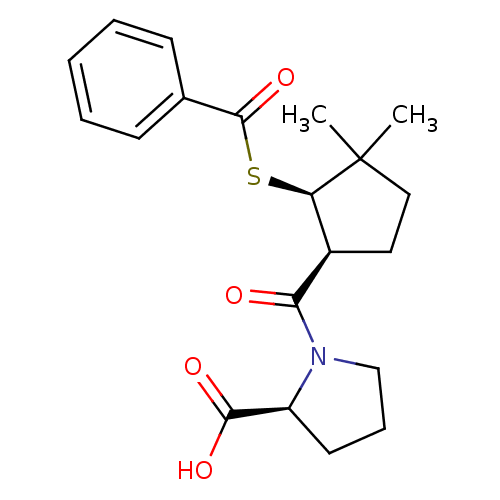 (1-(2-Benzoylsulfanyl-3,3-dimethyl-cyclopentanecarb...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021296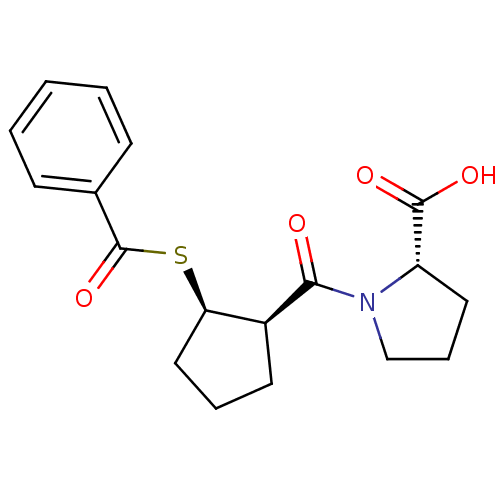 (1-(2-Benzoylsulfanyl-cyclopentanecarbonyl)-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50021300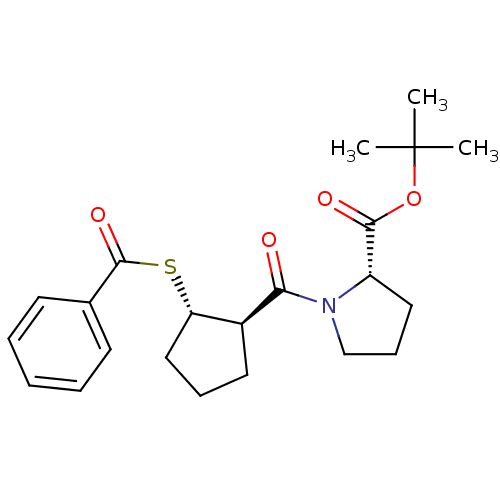 (1-(2-Benzoylsulfanyl-cyclopentanecarbonyl)-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin I converting enzyme | J Med Chem 29: 411-7 (1986) BindingDB Entry DOI: 10.7270/Q218372M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||