 Found 227 hits with Last Name = 'saarinen' and Initial = 'g'
Found 227 hits with Last Name = 'saarinen' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512929
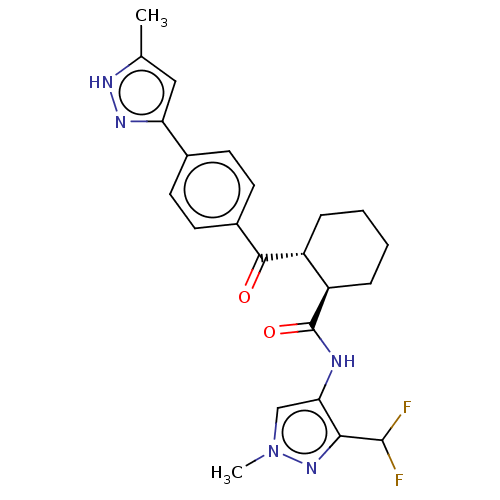
(CHEMBL4457556)Show SMILES Cc1cc(n[nH]1)-c1ccc(cc1)C(=O)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C(F)F |r| Show InChI InChI=1S/C23H25F2N5O2/c1-13-11-18(28-27-13)14-7-9-15(10-8-14)21(31)16-5-3-4-6-17(16)23(32)26-19-12-30(2)29-20(19)22(24)25/h7-12,16-17,22H,3-6H2,1-2H3,(H,26,32)(H,27,28)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... |
ACS Med Chem Lett 9: 594-599 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00505
BindingDB Entry DOI: 10.7270/Q2Z60RMJ |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512927

(CHEMBL4470157)Show SMILES [H][C@@]12C[C@]1([H])C[C@H]([C@@H](C2)C(=O)Nc1cn(C)nc1C(F)(F)F)C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C23H22F3N5O2/c1-31-11-19(21(30-31)23(24,25)26)28-22(33)17-10-15-8-14(15)9-16(17)20(32)13-4-2-12(3-5-13)18-6-7-27-29-18/h2-7,11,14-17H,8-10H2,1H3,(H,27,29)(H,28,33)/t14-,15+,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512917

(CHEMBL4527700)Show SMILES [H][C@@]12C[C@]1([H])C[C@H]([C@@H](C2)C(=O)Nc1cnn(C)c1C(F)(F)F)C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C23H22F3N5O2/c1-31-21(23(24,25)26)19(11-28-31)29-22(33)17-10-15-8-14(15)9-16(17)20(32)13-4-2-12(3-5-13)18-6-7-27-30-18/h2-7,11,14-17H,8-10H2,1H3,(H,27,30)(H,29,33)/t14-,15+,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458617

(CHEMBL4215835)Show SMILES CC(CC(O)=O)c1cccc(n1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(7-17(26)27)13-3-2-4-16(24-13)25-14-9-12(29-19(20,21)22)6-5-11(14)8-15(25)18(23)28/h2-6,8-10H,7H2,1H3,(H2,23,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458617

(CHEMBL4215835)Show SMILES CC(CC(O)=O)c1cccc(n1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(7-17(26)27)13-3-2-4-16(24-13)25-14-9-12(29-19(20,21)22)6-5-11(14)8-15(25)18(23)28/h2-6,8-10H,7H2,1H3,(H2,23,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458614

(CHEMBL4210991)Show SMILES C[C@@H](CC(O)=O)c1cccc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O |r| Show InChI InChI=1S/C20H17F3N2O4/c1-11(7-18(26)27)12-3-2-4-14(8-12)25-16-10-15(29-20(21,22)23)6-5-13(16)9-17(25)19(24)28/h2-6,8-11H,7H2,1H3,(H2,24,28)(H,26,27)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512909

(CHEMBL4528662)Show SMILES COc1cc(NC(=O)[C@@H]2CCCC[C@H]2C(=O)c2ccc(cc2)-c2cc[nH]n2)ncn1 |r| Show InChI InChI=1S/C22H23N5O3/c1-30-20-12-19(23-13-24-20)26-22(29)17-5-3-2-4-16(17)21(28)15-8-6-14(7-9-15)18-10-11-25-27-18/h6-13,16-17H,2-5H2,1H3,(H,25,27)(H,23,24,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512909

(CHEMBL4528662)Show SMILES COc1cc(NC(=O)[C@@H]2CCCC[C@H]2C(=O)c2ccc(cc2)-c2cc[nH]n2)ncn1 |r| Show InChI InChI=1S/C22H23N5O3/c1-30-20-12-19(23-13-24-20)26-22(29)17-5-3-2-4-16(17)21(28)15-8-6-14(7-9-15)18-10-11-25-27-18/h6-13,16-17H,2-5H2,1H3,(H,25,27)(H,23,24,26,29)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512914

(CHEMBL4471402)Show SMILES CC(C)(O)CNC(=O)c1cnc(NC(=O)[C@@H]2CCCC[C@H]2C(=O)c2ccc(cc2)-c2cc[nH]n2)cn1 |r| Show InChI InChI=1S/C26H30N6O4/c1-26(2,36)15-29-25(35)21-13-28-22(14-27-21)31-24(34)19-6-4-3-5-18(19)23(33)17-9-7-16(8-10-17)20-11-12-30-32-20/h7-14,18-19,36H,3-6,15H2,1-2H3,(H,29,35)(H,30,32)(H,28,31,34)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512928
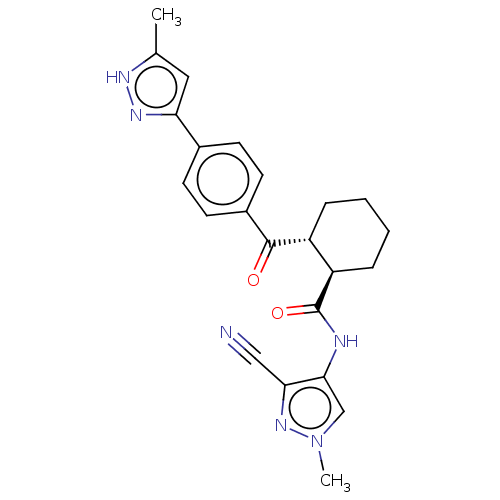
(CHEMBL4555790)Show SMILES Cc1cc(n[nH]1)-c1ccc(cc1)C(=O)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C#N |r| Show InChI InChI=1S/C23H24N6O2/c1-14-11-19(27-26-14)15-7-9-16(10-8-15)22(30)17-5-3-4-6-18(17)23(31)25-21-13-29(2)28-20(21)12-24/h7-11,13,17-18H,3-6H2,1-2H3,(H,25,31)(H,26,27)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512927

(CHEMBL4470157)Show SMILES [H][C@@]12C[C@]1([H])C[C@H]([C@@H](C2)C(=O)Nc1cn(C)nc1C(F)(F)F)C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C23H22F3N5O2/c1-31-11-19(21(30-31)23(24,25)26)28-22(33)17-10-15-8-14(15)9-16(17)20(32)13-4-2-12(3-5-13)18-6-7-27-29-18/h2-7,11,14-17H,8-10H2,1H3,(H,27,29)(H,28,33)/t14-,15+,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512916
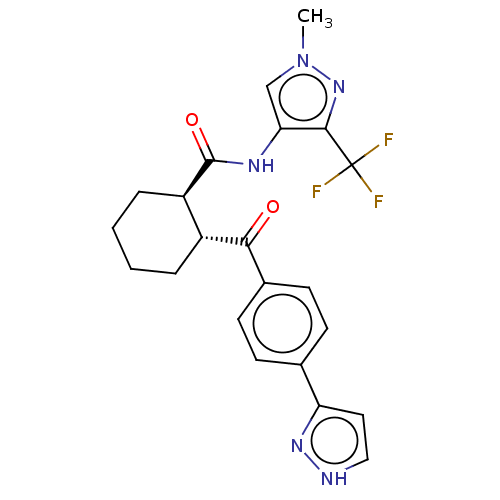
(CHEMBL4463314)Show SMILES Cn1cc(NC(=O)[C@@H]2CCCC[C@H]2C(=O)c2ccc(cc2)-c2cc[nH]n2)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C22H22F3N5O2/c1-30-12-18(20(29-30)22(23,24)25)27-21(32)16-5-3-2-4-15(16)19(31)14-8-6-13(7-9-14)17-10-11-26-28-17/h6-12,15-16H,2-5H2,1H3,(H,26,28)(H,27,32)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50366784
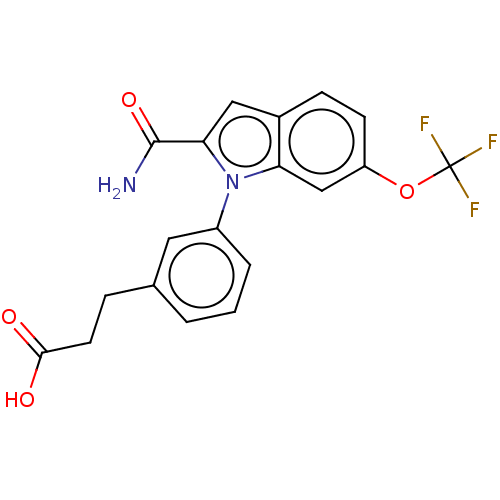
(CHEMBL4171084)Show SMILES NC(=O)c1cc2ccc(OC(F)(F)F)cc2n1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C19H15F3N2O4/c20-19(21,22)28-14-6-5-12-9-16(18(23)27)24(15(12)10-14)13-3-1-2-11(8-13)4-7-17(25)26/h1-3,5-6,8-10H,4,7H2,(H2,23,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50366784

(CHEMBL4171084)Show SMILES NC(=O)c1cc2ccc(OC(F)(F)F)cc2n1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C19H15F3N2O4/c20-19(21,22)28-14-6-5-12-9-16(18(23)27)24(15(12)10-14)13-3-1-2-11(8-13)4-7-17(25)26/h1-3,5-6,8-10H,4,7H2,(H2,23,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... |
ACS Med Chem Lett 9: 594-599 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00505
BindingDB Entry DOI: 10.7270/Q2Z60RMJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458605
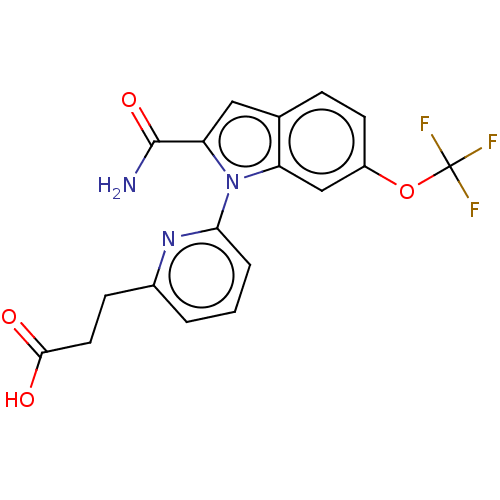
(CHEMBL4217510)Show SMILES NC(=O)c1cc2ccc(OC(F)(F)F)cc2n1-c1cccc(CCC(O)=O)n1 Show InChI InChI=1S/C18H14F3N3O4/c19-18(20,21)28-12-6-4-10-8-14(17(22)27)24(13(10)9-12)15-3-1-2-11(23-15)5-7-16(25)26/h1-4,6,8-9H,5,7H2,(H2,22,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512910

(CHEMBL4438207)Show SMILES Cn1cc(NC(=O)[C@@H]2CCCC[C@H]2C(=O)c2ccc(cc2)-c2cc[nH]n2)c(OC(F)F)n1 |r| Show InChI InChI=1S/C22H23F2N5O3/c1-29-12-18(21(28-29)32-22(23)24)26-20(31)16-5-3-2-4-15(16)19(30)14-8-6-13(7-9-14)17-10-11-25-27-17/h6-12,15-16,22H,2-5H2,1H3,(H,25,27)(H,26,31)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458612
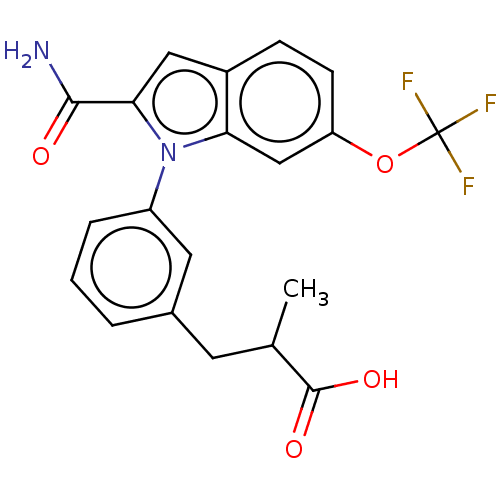
(CHEMBL4214052)Show SMILES CC(Cc1cccc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C20H17F3N2O4/c1-11(19(27)28)7-12-3-2-4-14(8-12)25-16-10-15(29-20(21,22)23)6-5-13(16)9-17(25)18(24)26/h2-6,8-11H,7H2,1H3,(H2,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458612

(CHEMBL4214052)Show SMILES CC(Cc1cccc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C20H17F3N2O4/c1-11(19(27)28)7-12-3-2-4-14(8-12)25-16-10-15(29-20(21,22)23)6-5-13(16)9-17(25)18(24)26/h2-6,8-11H,7H2,1H3,(H2,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512922

(CHEMBL4533700)Show SMILES O=C(Nc1cnn2cccnc12)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C23H22N6O2/c30-21(16-8-6-15(7-9-16)19-10-12-25-28-19)17-4-1-2-5-18(17)23(31)27-20-14-26-29-13-3-11-24-22(20)29/h3,6-14,17-18H,1-2,4-5H2,(H,25,28)(H,27,31)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458613
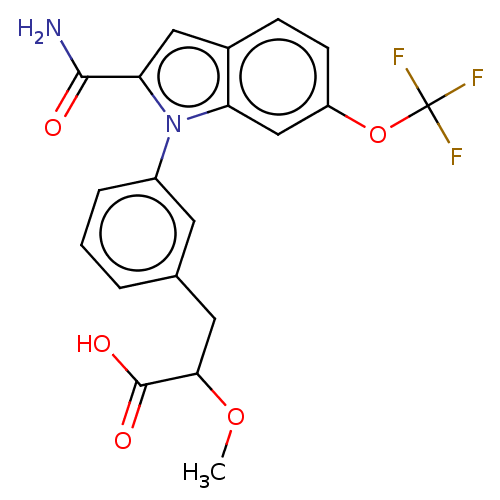
(CHEMBL4204172)Show SMILES COC(Cc1cccc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C20H17F3N2O5/c1-29-17(19(27)28)8-11-3-2-4-13(7-11)25-15-10-14(30-20(21,22)23)6-5-12(15)9-16(25)18(24)26/h2-7,9-10,17H,8H2,1H3,(H2,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458613

(CHEMBL4204172)Show SMILES COC(Cc1cccc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C20H17F3N2O5/c1-29-17(19(27)28)8-11-3-2-4-13(7-11)25-15-10-14(30-20(21,22)23)6-5-12(15)9-16(25)18(24)26/h2-7,9-10,17H,8H2,1H3,(H2,24,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512922

(CHEMBL4533700)Show SMILES O=C(Nc1cnn2cccnc12)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C23H22N6O2/c30-21(16-8-6-15(7-9-16)19-10-12-25-28-19)17-4-1-2-5-18(17)23(31)27-20-14-26-29-13-3-11-24-22(20)29/h3,6-14,17-18H,1-2,4-5H2,(H,25,28)(H,27,31)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512912
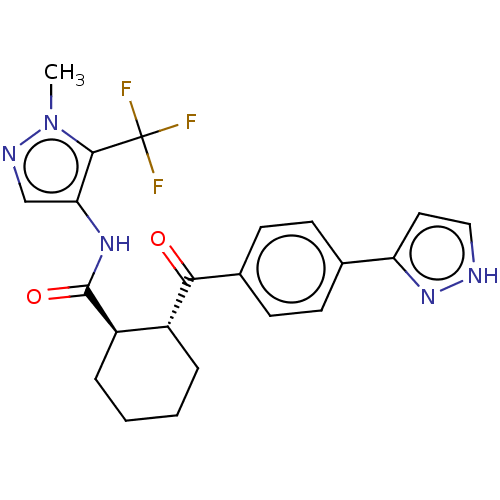
(CHEMBL4534060)Show SMILES Cn1ncc(NC(=O)[C@@H]2CCCC[C@H]2C(=O)c2ccc(cc2)-c2cc[nH]n2)c1C(F)(F)F |r| Show InChI InChI=1S/C22H22F3N5O2/c1-30-20(22(23,24)25)18(12-27-30)28-21(32)16-5-3-2-4-15(16)19(31)14-8-6-13(7-9-14)17-10-11-26-29-17/h6-12,15-16H,2-5H2,1H3,(H,26,29)(H,28,32)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512937
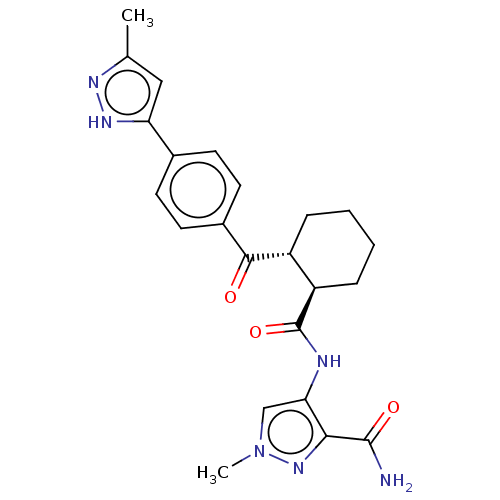
(CHEMBL4450838)Show SMILES Cc1cc([nH]n1)-c1ccc(cc1)C(=O)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C(N)=O |r| Show InChI InChI=1S/C23H26N6O3/c1-13-11-18(27-26-13)14-7-9-15(10-8-14)21(30)16-5-3-4-6-17(16)23(32)25-19-12-29(2)28-20(19)22(24)31/h7-12,16-17H,3-6H2,1-2H3,(H2,24,31)(H,25,32)(H,26,27)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512913
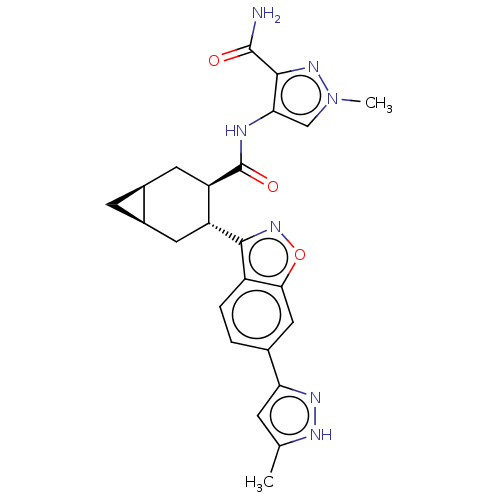
(CHEMBL4562028)Show SMILES [H][C@@]12C[C@]1([H])C[C@H]([C@@H](C2)C(=O)Nc1cn(C)nc1C(N)=O)c1noc2cc(ccc12)-c1cc(C)[nH]n1 |r| Show InChI InChI=1S/C24H25N7O3/c1-11-5-18(28-27-11)12-3-4-15-20(9-12)34-30-21(15)16-7-13-6-14(13)8-17(16)24(33)26-19-10-31(2)29-22(19)23(25)32/h3-5,9-10,13-14,16-17H,6-8H2,1-2H3,(H2,25,32)(H,26,33)(H,27,28)/t13-,14+,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... |
ACS Med Chem Lett 9: 594-599 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00505
BindingDB Entry DOI: 10.7270/Q2Z60RMJ |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458617

(CHEMBL4215835)Show SMILES CC(CC(O)=O)c1cccc(n1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(7-17(26)27)13-3-2-4-16(24-13)25-14-9-12(29-19(20,21)22)6-5-11(14)8-15(25)18(23)28/h2-6,8-10H,7H2,1H3,(H2,23,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50366811
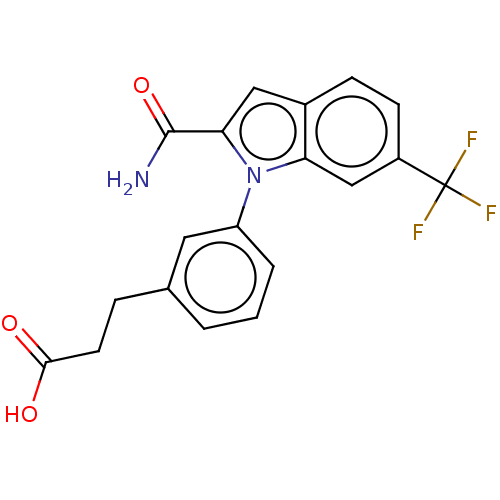
(CHEMBL4176544)Show SMILES NC(=O)c1cc2ccc(cc2n1-c1cccc(CCC(O)=O)c1)C(F)(F)F Show InChI InChI=1S/C19H15F3N2O3/c20-19(21,22)13-6-5-12-9-16(18(23)27)24(15(12)10-13)14-3-1-2-11(8-14)4-7-17(25)26/h1-3,5-6,8-10H,4,7H2,(H2,23,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... |
ACS Med Chem Lett 9: 594-599 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00505
BindingDB Entry DOI: 10.7270/Q2Z60RMJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50458608
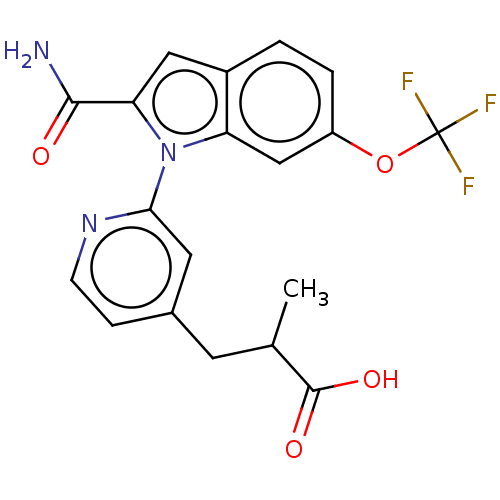
(CHEMBL4205008)Show SMILES CC(Cc1ccnc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(18(27)28)6-11-4-5-24-16(7-11)25-14-9-13(29-19(20,21)22)3-2-12(14)8-15(25)17(23)26/h2-5,7-10H,6H2,1H3,(H2,23,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50458608

(CHEMBL4205008)Show SMILES CC(Cc1ccnc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(18(27)28)6-11-4-5-24-16(7-11)25-14-9-13(29-19(20,21)22)3-2-12(14)8-15(25)17(23)26/h2-5,7-10H,6H2,1H3,(H2,23,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512917

(CHEMBL4527700)Show SMILES [H][C@@]12C[C@]1([H])C[C@H]([C@@H](C2)C(=O)Nc1cnn(C)c1C(F)(F)F)C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C23H22F3N5O2/c1-31-21(23(24,25)26)19(11-28-31)29-22(33)17-10-15-8-14(15)9-16(17)20(32)13-4-2-12(3-5-13)18-6-7-27-30-18/h2-7,11,14-17H,8-10H2,1H3,(H,27,30)(H,29,33)/t14-,15+,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512916

(CHEMBL4463314)Show SMILES Cn1cc(NC(=O)[C@@H]2CCCC[C@H]2C(=O)c2ccc(cc2)-c2cc[nH]n2)c(n1)C(F)(F)F |r| Show InChI InChI=1S/C22H22F3N5O2/c1-30-12-18(20(29-30)22(23,24)25)27-21(32)16-5-3-2-4-15(16)19(31)14-8-6-13(7-9-14)17-10-11-26-28-17/h6-12,15-16H,2-5H2,1H3,(H,26,28)(H,27,32)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512912

(CHEMBL4534060)Show SMILES Cn1ncc(NC(=O)[C@@H]2CCCC[C@H]2C(=O)c2ccc(cc2)-c2cc[nH]n2)c1C(F)(F)F |r| Show InChI InChI=1S/C22H22F3N5O2/c1-30-20(22(23,24)25)18(12-27-30)28-21(32)16-5-3-2-4-15(16)19(31)14-8-6-13(7-9-14)17-10-11-26-29-17/h6-12,15-16H,2-5H2,1H3,(H,26,29)(H,28,32)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512926
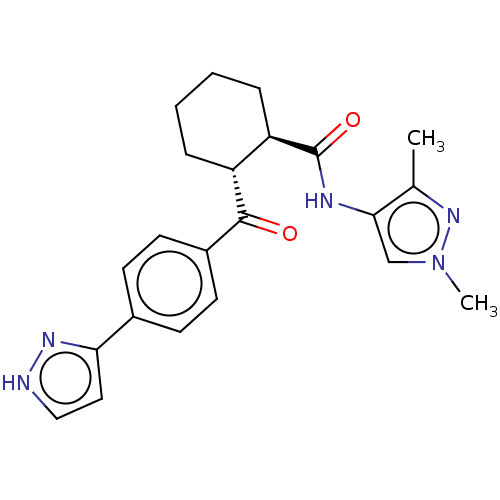
(CHEMBL4554389)Show SMILES Cc1nn(C)cc1NC(=O)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C22H25N5O2/c1-14-20(13-27(2)26-14)24-22(29)18-6-4-3-5-17(18)21(28)16-9-7-15(8-10-16)19-11-12-23-25-19/h7-13,17-18H,3-6H2,1-2H3,(H,23,25)(H,24,29)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512926

(CHEMBL4554389)Show SMILES Cc1nn(C)cc1NC(=O)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C22H25N5O2/c1-14-20(13-27(2)26-14)24-22(29)18-6-4-3-5-17(18)21(28)16-9-7-15(8-10-16)19-11-12-23-25-19/h7-13,17-18H,3-6H2,1-2H3,(H,23,25)(H,24,29)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50458612

(CHEMBL4214052)Show SMILES CC(Cc1cccc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C20H17F3N2O4/c1-11(19(27)28)7-12-3-2-4-14(8-12)25-16-10-15(29-20(21,22)23)6-5-13(16)9-17(25)18(24)26/h2-6,8-11H,7H2,1H3,(H2,24,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Phospholipase A2, membrane associated
(Homo sapiens (Human)) | BDBM50458612

(CHEMBL4214052)Show SMILES CC(Cc1cccc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C20H17F3N2O4/c1-11(19(27)28)7-12-3-2-4-14(8-12)25-16-10-15(29-20(21,22)23)6-5-13(16)9-17(25)18(24)26/h2-6,8-11H,7H2,1H3,(H2,24,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512930
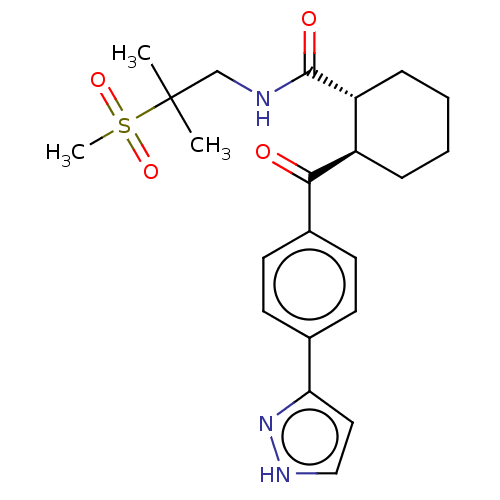
(CHEMBL4589058)Show SMILES CC(C)(CNC(=O)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1)-c1cc[nH]n1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H29N3O4S/c1-22(2,30(3,28)29)14-23-21(27)18-7-5-4-6-17(18)20(26)16-10-8-15(9-11-16)19-12-13-24-25-19/h8-13,17-18H,4-7,14H2,1-3H3,(H,23,27)(H,24,25)/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512931

(CHEMBL4528928)Show SMILES Cc1cc(n[nH]1)-c1ccc(cc1)C(=O)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C |r| Show InChI InChI=1S/C23H27N5O2/c1-14-12-20(26-25-14)16-8-10-17(11-9-16)22(29)18-6-4-5-7-19(18)23(30)24-21-13-28(3)27-15(21)2/h8-13,18-19H,4-7H2,1-3H3,(H,24,30)(H,25,26)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512936
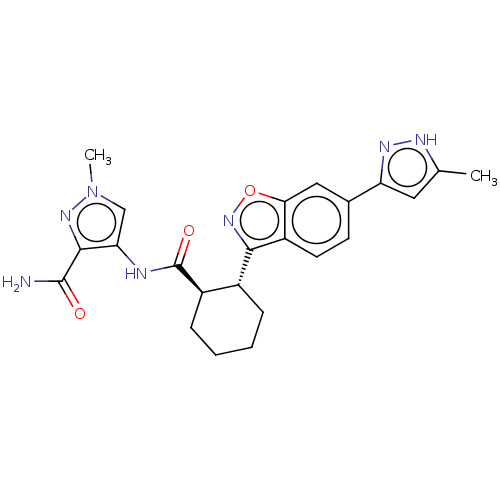
(CHEMBL4472842)Show SMILES Cc1cc(n[nH]1)-c1ccc2c(noc2c1)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C(N)=O |r| Show InChI InChI=1S/C23H25N7O3/c1-12-9-17(27-26-12)13-7-8-16-19(10-13)33-29-20(16)14-5-3-4-6-15(14)23(32)25-18-11-30(2)28-21(18)22(24)31/h7-11,14-15H,3-6H2,1-2H3,(H2,24,31)(H,25,32)(H,26,27)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 4 hrs followed by calc... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512931

(CHEMBL4528928)Show SMILES Cc1cc(n[nH]1)-c1ccc(cc1)C(=O)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C |r| Show InChI InChI=1S/C23H27N5O2/c1-14-12-20(26-25-14)16-8-10-17(11-9-16)22(29)18-6-4-5-7-19(18)23(30)24-21-13-28(3)27-15(21)2/h8-13,18-19H,4-7H2,1-3H3,(H,24,30)(H,25,26)/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512929

(CHEMBL4457556)Show SMILES Cc1cc(n[nH]1)-c1ccc(cc1)C(=O)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C(F)F |r| Show InChI InChI=1S/C23H25F2N5O2/c1-13-11-18(28-27-13)14-7-9-15(10-8-14)21(31)16-5-3-4-6-17(16)23(32)26-19-12-30(2)29-20(19)22(24)25/h7-12,16-17,22H,3-6H2,1-2H3,(H,26,32)(H,27,28)/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512918

(CHEMBL4458304)Show SMILES [H][C@]12CC[C@]([H])(CC1)[C@H]([C@@H]2C(=O)Nc1cn(C)nc1C)C(=O)c1ccc(cc1F)-c1cc[nH]n1 |r,wU:9.11,4.4,1.0,wD:8.22,(51.99,-6.95,;51.99,-8.49,;50.66,-9.28,;50.66,-10.83,;51.99,-11.59,;51.99,-13.13,;51.36,-10.26,;52.48,-9.82,;53.33,-10.83,;53.33,-9.28,;54.67,-8.51,;56.01,-9.29,;54.68,-6.96,;56.02,-6.19,;56.17,-4.66,;57.68,-4.34,;58.31,-2.94,;58.45,-5.68,;57.41,-6.82,;57.73,-8.33,;54.67,-11.6,;56.01,-10.83,;54.67,-13.13,;53.33,-13.9,;53.33,-15.44,;54.67,-16.21,;56.01,-15.44,;56,-13.9,;57.33,-13.13,;54.68,-17.75,;53.43,-18.66,;53.91,-20.12,;55.45,-20.12,;55.93,-18.66,)| Show InChI InChI=1S/C24H26FN5O2/c1-13-20(12-30(2)29-13)27-24(32)22-15-5-3-14(4-6-15)21(22)23(31)17-8-7-16(11-18(17)25)19-9-10-26-28-19/h7-12,14-15,21-22H,3-6H2,1-2H3,(H,26,28)(H,27,32)/t14-,15+,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512919
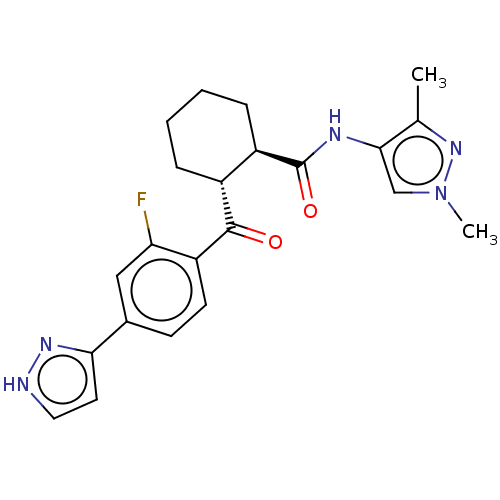
(CHEMBL4554158)Show SMILES Cc1nn(C)cc1NC(=O)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1F)-c1cc[nH]n1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-13-20(12-28(2)27-13)25-22(30)16-6-4-3-5-15(16)21(29)17-8-7-14(11-18(17)23)19-9-10-24-26-19/h7-12,15-16H,3-6H2,1-2H3,(H,24,26)(H,25,30)/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458606
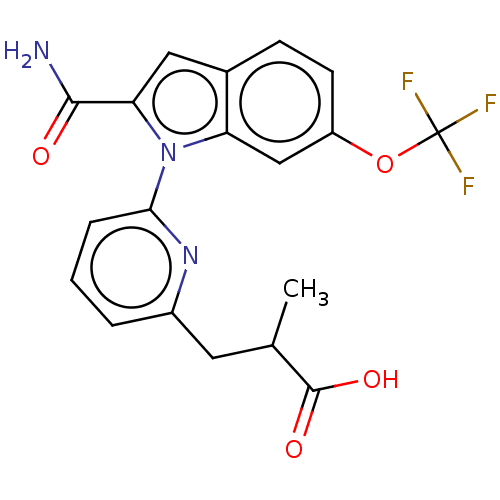
(CHEMBL4205511)Show SMILES CC(Cc1cccc(n1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(18(27)28)7-12-3-2-4-16(24-12)25-14-9-13(29-19(20,21)22)6-5-11(14)8-15(25)17(23)26/h2-6,8-10H,7H2,1H3,(H2,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50458606

(CHEMBL4205511)Show SMILES CC(Cc1cccc(n1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(18(27)28)7-12-3-2-4-16(24-12)25-14-9-13(29-19(20,21)22)6-5-11(14)8-15(25)17(23)26/h2-6,8-10H,7H2,1H3,(H2,23,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50366983
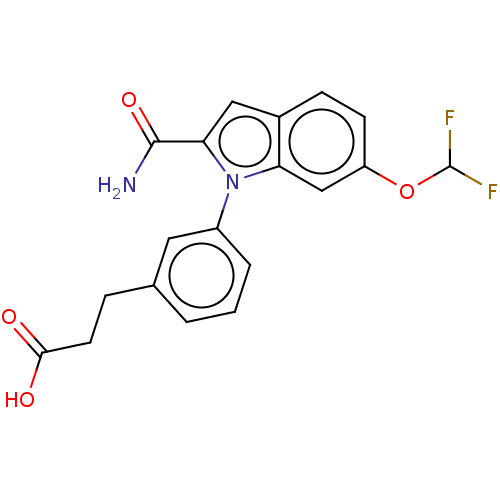
(CHEMBL4160483)Show SMILES NC(=O)c1cc2ccc(OC(F)F)cc2n1-c1cccc(CCC(O)=O)c1 Show InChI InChI=1S/C19H16F2N2O4/c20-19(21)27-14-6-5-12-9-16(18(22)26)23(15(12)10-14)13-3-1-2-11(8-13)4-7-17(24)25/h1-3,5-6,8-10,19H,4,7H2,(H2,22,26)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... |
ACS Med Chem Lett 9: 594-599 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00505
BindingDB Entry DOI: 10.7270/Q2Z60RMJ |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Mus musculus) | BDBM50458617

(CHEMBL4215835)Show SMILES CC(CC(O)=O)c1cccc(n1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(7-17(26)27)13-3-2-4-16(24-13)25-14-9-12(29-19(20,21)22)6-5-11(14)8-15(25)18(23)28/h2-6,8-10H,7H2,1H3,(H2,23,28)(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse sPLA2-10 |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Arachidonate 5-lipoxygenase-activating protein
(Homo sapiens (Human)) | BDBM50512936

(CHEMBL4472842)Show SMILES Cc1cc(n[nH]1)-c1ccc2c(noc2c1)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C(N)=O |r| Show InChI InChI=1S/C23H25N7O3/c1-12-9-17(27-26-12)13-7-8-16-19(10-13)33-29-20(16)14-5-3-4-6-15(14)23(32)25-18-11-30(2)28-21(18)22(24)31/h7-11,14-15H,3-6H2,1-2H3,(H2,24,31)(H,25,32)(H,26,27)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data