 Found 163 hits with Last Name = 'pauli' and Initial = 'gf'
Found 163 hits with Last Name = 'pauli' and Initial = 'gf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPDPE from human delta opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPDPE from human delta opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50292340
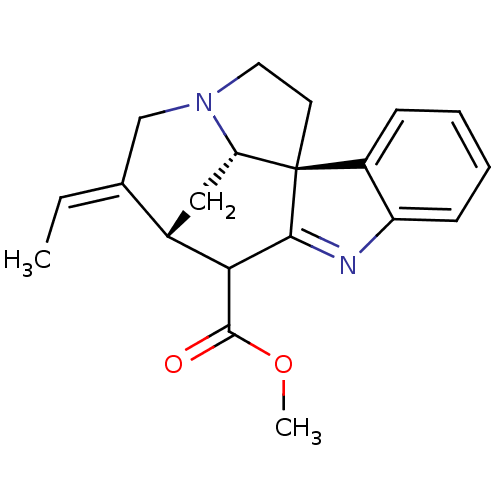
(AKUAMMICINE | CHEMBL508955)Show SMILES COC(=O)C1[C@H]2C[C@@H]3N(CC[C@]33C1=Nc1ccccc31)C\C2=C\C |r,t:14| Show InChI InChI=1S/C20H22N2O2/c1-3-12-11-22-9-8-20-14-6-4-5-7-15(14)21-18(20)17(19(23)24-2)13(12)10-16(20)22/h3-7,13,16-17H,8-11H2,1-2H3/b12-3-/t13-,16-,17?,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50292340

(AKUAMMICINE | CHEMBL508955)Show SMILES COC(=O)C1[C@H]2C[C@@H]3N(CC[C@]33C1=Nc1ccccc31)C\C2=C\C |r,t:14| Show InChI InChI=1S/C20H22N2O2/c1-3-12-11-22-9-8-20-14-6-4-5-7-15(14)21-18(20)17(19(23)24-2)13(12)10-16(20)22/h3-7,13,16-17H,8-11H2,1-2H3/b12-3-/t13-,16-,17?,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant CYP1A1 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A5
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant CYP3A5 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50174315
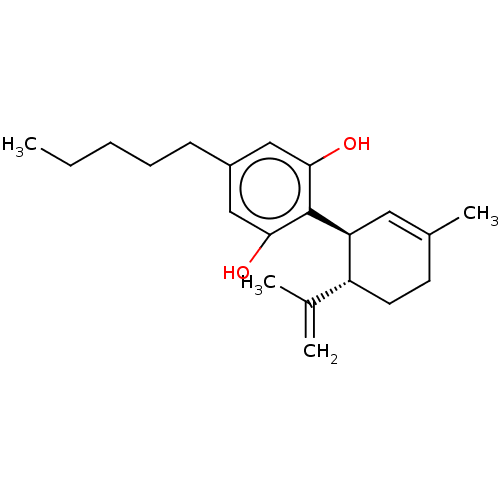
(CHEMBL3810140)Show SMILES CCCCCc1cc(O)c([C@H]2C=C(C)CC[C@@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-HU-243 from CB2 receptor (unknown origin) expressed in African green monkey Cos7 cell membranes incubated for 90 mins by radioli... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50568638

(CHEMBL4871880)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5cc(O)ccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:28:5:2:10.20.23,11:10:2:7.5.6,21:20:2:7.5.6,18:20:2:7.5.6,22:23:2:7.5.6,THB:6:7:20:2.23.3,24:23:2:7.5.6,8:7:2:10.20.23,9:10:2:7.5.6| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50568642

(CHEMBL4856018)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1[C@@]([H])(Cc1c2[nH]c2ccccc12)[C@@]3(CO)C(=O)OC)=CC |r,TLB:10:8:1.2:5.6,THB:21:20:1.2:5.6,6:7:11.12.10:20.2.3,23:20:1.2:5.6,12:1:20.8:5.6| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50568638

(CHEMBL4871880)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5cc(O)ccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:28:5:2:10.20.23,11:10:2:7.5.6,21:20:2:7.5.6,18:20:2:7.5.6,22:23:2:7.5.6,THB:6:7:20:2.23.3,24:23:2:7.5.6,8:7:2:10.20.23,9:10:2:7.5.6| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50568642

(CHEMBL4856018)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1[C@@]([H])(Cc1c2[nH]c2ccccc12)[C@@]3(CO)C(=O)OC)=CC |r,TLB:10:8:1.2:5.6,THB:21:20:1.2:5.6,6:7:11.12.10:20.2.3,23:20:1.2:5.6,12:1:20.8:5.6| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50568639

(CHEMBL4846243)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5ccccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:27:5:2:10.19.22,11:10:2:7.5.6,20:19:2:7.5.6,21:22:2:7.5.6,17:19:2:7.5.6,THB:6:7:19:2.22.3,23:22:2:7.5.6,8:7:2:10.19.22,9:10:2:7.5.6| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50568639

(CHEMBL4846243)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5ccccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:27:5:2:10.19.22,11:10:2:7.5.6,20:19:2:7.5.6,21:22:2:7.5.6,17:19:2:7.5.6,THB:6:7:19:2.22.3,23:22:2:7.5.6,8:7:2:10.19.22,9:10:2:7.5.6| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of human recombinant CYP2B6 expressed in baculovirus-infected insect cells using coumarin as substrate preincubated for 5 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of human recombinant CYP2C19 using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH-generating system add... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Rattus norvegicus (rat)) | BDBM50174315

(CHEMBL3810140)Show SMILES CCCCCc1cc(O)c([C@H]2C=C(C)CC[C@@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes incubated for 90 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant CYP3A4 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of 5-HT2C (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50568640

(CHEMBL4874877)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]1(C2=Nc2ccccc12)[C@]3(COC(C)=O)C(=O)OC)=CC |r,t:13,TLB:29:5:2:10.11.19,18:10:2:7.5.6,12:11:2:7.5.6,20:19:2:7.5.6,THB:25:19:2:7.5.6,8:7:2:10.11.19,9:10:2:7.5.6,6:7:11:2.19.3| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50568640

(CHEMBL4874877)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]1(C2=Nc2ccccc12)[C@]3(COC(C)=O)C(=O)OC)=CC |r,t:13,TLB:29:5:2:10.11.19,18:10:2:7.5.6,12:11:2:7.5.6,20:19:2:7.5.6,THB:25:19:2:7.5.6,8:7:2:10.11.19,9:10:2:7.5.6,6:7:11:2.19.3| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mu-type opioid receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50568638

(CHEMBL4871880)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5cc(O)ccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:28:5:2:10.20.23,11:10:2:7.5.6,21:20:2:7.5.6,18:20:2:7.5.6,22:23:2:7.5.6,THB:6:7:20:2.23.3,24:23:2:7.5.6,8:7:2:10.20.23,9:10:2:7.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50568638

(CHEMBL4871880)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5cc(O)ccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:28:5:2:10.20.23,11:10:2:7.5.6,21:20:2:7.5.6,18:20:2:7.5.6,22:23:2:7.5.6,THB:6:7:20:2.23.3,24:23:2:7.5.6,8:7:2:10.20.23,9:10:2:7.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50568639

(CHEMBL4846243)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5ccccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:27:5:2:10.19.22,11:10:2:7.5.6,20:19:2:7.5.6,21:22:2:7.5.6,17:19:2:7.5.6,THB:6:7:19:2.22.3,23:22:2:7.5.6,8:7:2:10.19.22,9:10:2:7.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50568639

(CHEMBL4846243)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5ccccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:27:5:2:10.19.22,11:10:2:7.5.6,20:19:2:7.5.6,21:22:2:7.5.6,17:19:2:7.5.6,THB:6:7:19:2.22.3,23:22:2:7.5.6,8:7:2:10.19.22,9:10:2:7.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of kappa-type opioid receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50568641
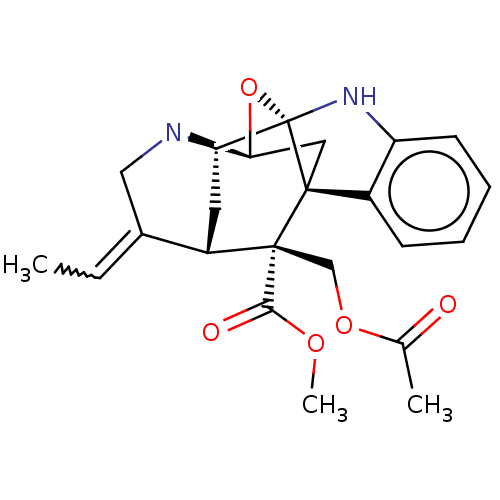
(CHEMBL4852987)Show SMILES [H][C@@]12C[C@@]34c5ccccc5N[C@]3(O1)[C@]1([H])C[C@@]([H])(C(CN21)=CC)[C@@]4(COC(C)=O)C(=O)OC |r,TLB:21:18:15:3.11.23,4:3:15:20.18.19,10:11:15:20.18.19,24:23:15:20.18.19,THB:29:23:15:20.18.19,1:20:15:3.11.23,2:3:15:20.18.19,12:11:15:20.18.19| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 10 mins in presence of NADPH generating by Lineweave... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50568641

(CHEMBL4852987)Show SMILES [H][C@@]12C[C@@]34c5ccccc5N[C@]3(O1)[C@]1([H])C[C@@]([H])(C(CN21)=CC)[C@@]4(COC(C)=O)C(=O)OC |r,TLB:21:18:15:3.11.23,4:3:15:20.18.19,10:11:15:20.18.19,24:23:15:20.18.19,THB:29:23:15:20.18.19,1:20:15:3.11.23,2:3:15:20.18.19,12:11:15:20.18.19| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant CYP1A2 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of D1 dopamine receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of histamine H3 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50292340

(AKUAMMICINE | CHEMBL508955)Show SMILES COC(=O)C1[C@H]2C[C@@H]3N(CC[C@]33C1=Nc1ccccc31)C\C2=C\C |r,t:14| Show InChI InChI=1S/C20H22N2O2/c1-3-12-11-22-9-8-20-14-6-4-5-7-15(14)21-18(20)17(19(23)24-2)13(12)10-16(20)22/h3-7,13,16-17H,8-11H2,1-2H3/b12-3-/t13-,16-,17?,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alpha2B receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50292340

(AKUAMMICINE | CHEMBL508955)Show SMILES COC(=O)C1[C@H]2C[C@@H]3N(CC[C@]33C1=Nc1ccccc31)C\C2=C\C |r,t:14| Show InChI InChI=1S/C20H22N2O2/c1-3-12-11-22-9-8-20-14-6-4-5-7-15(14)21-18(20)17(19(23)24-2)13(12)10-16(20)22/h3-7,13,16-17H,8-11H2,1-2H3/b12-3-/t13-,16-,17?,20+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Sigma intracellular receptor 2
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of sigma2 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of human recombinant CYP1B1 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of alpha2C receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of CYP2C9 in pooled human liver microsomes using S-warfarin as substrate preincubated for 5 mins followed by NADPH-generating ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of delta-type opioid receptor receptor (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50568639

(CHEMBL4846243)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5ccccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:27:5:2:10.19.22,11:10:2:7.5.6,20:19:2:7.5.6,21:22:2:7.5.6,17:19:2:7.5.6,THB:6:7:19:2.22.3,23:22:2:7.5.6,8:7:2:10.19.22,9:10:2:7.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPDPE from human delta opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50568639

(CHEMBL4846243)Show SMILES [H][C@@]12C[C@@]3([H])C(CN1CC[C@@]14c5ccccc5N(C)[C@@]21OC[C@]34C(=O)OC)=CC |r,TLB:27:5:2:10.19.22,11:10:2:7.5.6,20:19:2:7.5.6,21:22:2:7.5.6,17:19:2:7.5.6,THB:6:7:19:2.22.3,23:22:2:7.5.6,8:7:2:10.19.22,9:10:2:7.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPDPE from human delta opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data