

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50478894 (Anabaenopeptin G) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A (unknown origin) assessed as reduction in hydrolysis of hippuryl-L-phenylalanine using hippuryl-L-phenylalanine as s... | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Bos taurus (bovine)) | BDBM50478892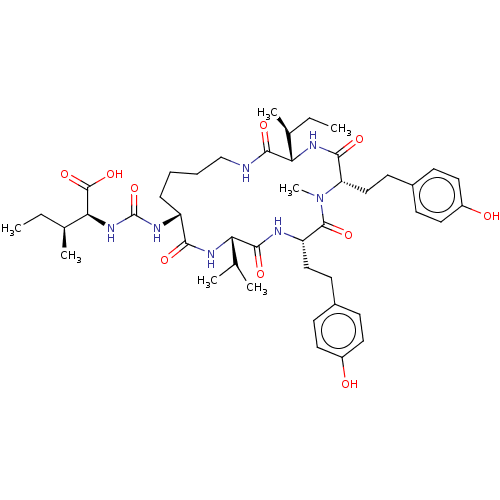 (Anabaenopeptin T) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A in bovine pancreas assessed as reduction in hydrolysis of hippuryl-L-phenylalanine using hippuryl-L-phenylalanine as... | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50478893 (Anabaenopeptin H) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A (unknown origin) assessed as reduction in hydrolysis of hippuryl-L-phenylalanine using hippuryl-L-phenylalanine as s... | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50558425 (CHEMBL4746522) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A (unknown origin) assessed as reduction in degradation of N-(4-methoxyphenylazoformyl)-Phe-OH using N-(4-methoxypheny... | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-arachidonyl glycine receptor (Homo sapiens (Human)) | BDBM50448075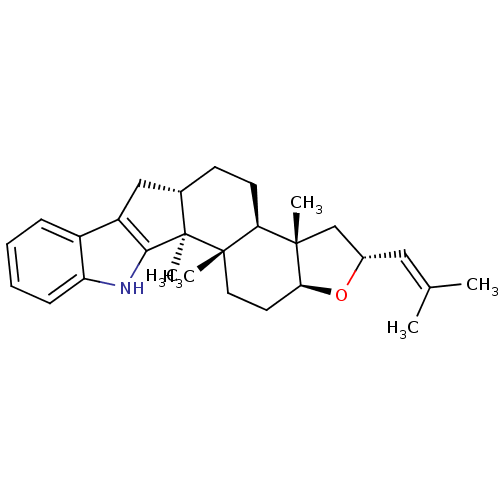 (CHEMBL1258979) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inverse agonist activity at human GPR18 expressed in CHO cells assessed as inhibition of 7.5 uM THC-mediated beta-arrestin recruitment after 90 mins ... | J Nat Prod 77: 673-7 (2014) Article DOI: 10.1021/np400850g BindingDB Entry DOI: 10.7270/Q2154JJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM50448073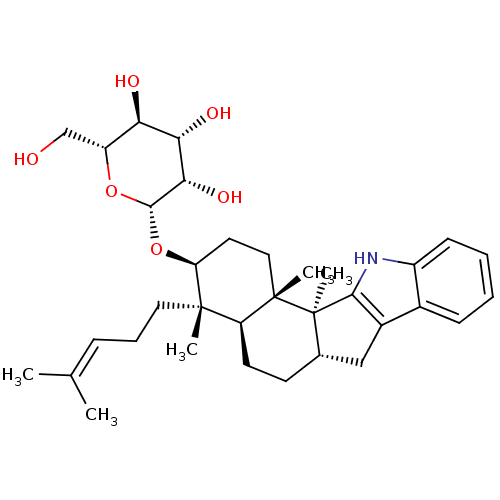 (CHEMBL3120631) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human GPR55 expressed in CHO cells assessed as inhibition of LPI-mediated beta-arrestin recruitment after 90 mins by beta-gala... | J Nat Prod 77: 673-7 (2014) Article DOI: 10.1021/np400850g BindingDB Entry DOI: 10.7270/Q2154JJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM50448076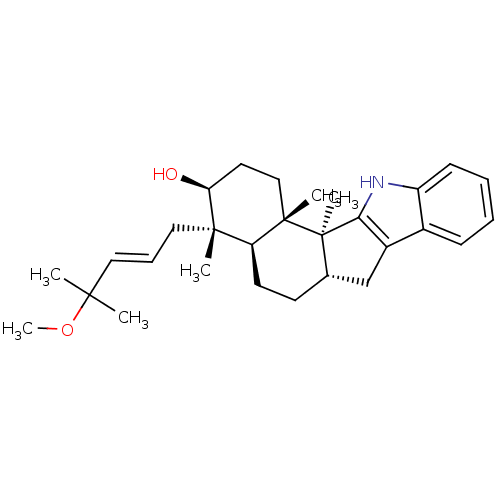 (CHEMBL3120632) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human GPR55 expressed in CHO cells assessed as inhibition of LPI-mediated beta-arrestin recruitment after 90 mins by beta-gala... | J Nat Prod 77: 673-7 (2014) Article DOI: 10.1021/np400850g BindingDB Entry DOI: 10.7270/Q2154JJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM50448075 (CHEMBL1258979) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human GPR55 expressed in CHO cells assessed as inhibition of LPI-mediated beta-arrestin recruitment after 90 mins by beta-gala... | J Nat Prod 77: 673-7 (2014) Article DOI: 10.1021/np400850g BindingDB Entry DOI: 10.7270/Q2154JJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 55 (Homo sapiens (Human)) | BDBM50448074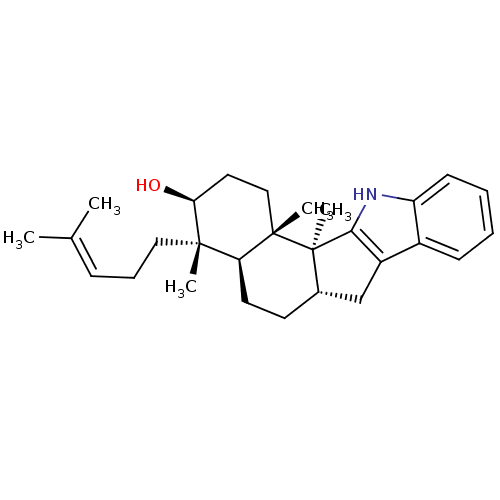 (Emindole Sb) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human GPR55 expressed in CHO cells assessed as inhibition of LPI-mediated beta-arrestin recruitment after 90 mins by beta-gala... | J Nat Prod 77: 673-7 (2014) Article DOI: 10.1021/np400850g BindingDB Entry DOI: 10.7270/Q2154JJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-arachidonyl glycine receptor (Homo sapiens (Human)) | BDBM50448073 (CHEMBL3120631) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human GPR18 expressed in CHO cells assessed as inhibition of 10 uM THC-mediated beta-arrestin recruitment after 90 mins by bet... | J Nat Prod 77: 673-7 (2014) Article DOI: 10.1021/np400850g BindingDB Entry DOI: 10.7270/Q2154JJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-arachidonyl glycine receptor (Homo sapiens (Human)) | BDBM50448074 (Emindole Sb) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Antagonist activity at human GPR18 expressed in CHO cells assessed as inhibition of 10 uM THC-mediated beta-arrestin recruitment after 90 mins by bet... | J Nat Prod 77: 673-7 (2014) Article DOI: 10.1021/np400850g BindingDB Entry DOI: 10.7270/Q2154JJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50478893 (Anabaenopeptin H) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A (unknown origin) assessed as reduction in degradation of N-(4-methoxyphenylazoformyl)-Phe-OH using N-(4-methoxypheny... | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50558424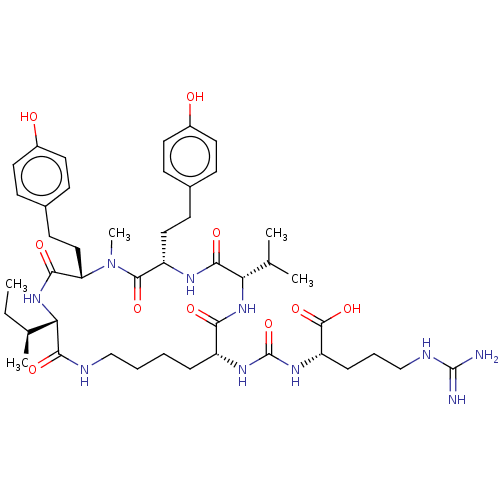 (Anabaenopeptin 908) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A (unknown origin) assessed as reduction in degradation of N-(4-methoxyphenylazoformyl)-Phe-OH using N-(4-methoxypheny... | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-arachidonyl glycine receptor (Homo sapiens (Human)) | BDBM50448076 (CHEMBL3120632) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inverse agonist activity at human GPR18 expressed in CHO cells assessed as inhibition of 7.5 uM THC-mediated beta-arrestin recruitment after 90 mins ... | J Nat Prod 77: 673-7 (2014) Article DOI: 10.1021/np400850g BindingDB Entry DOI: 10.7270/Q2154JJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50558424 (Anabaenopeptin 908) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50089688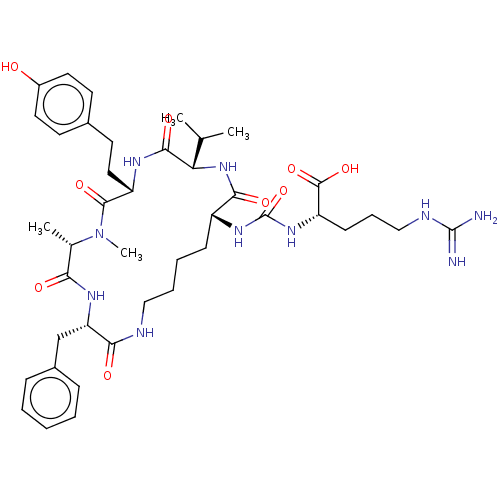 (ANABAENOPEPTIN B) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A (unknown origin) assessed as reduction in degradation of N-(4-methoxyphenylazoformyl)-Phe-OH using N-(4-methoxypheny... | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase A1 (Homo sapiens (Human)) | BDBM50089687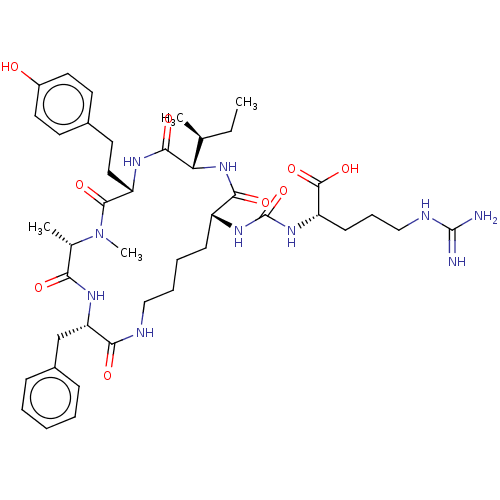 (Anabaenopeptin F) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of carboxypeptidase A (unknown origin) assessed as reduction in degradation of N-(4-methoxyphenylazoformyl)-Phe-OH using N-(4-methoxypheny... | Citation and Details Article DOI: 10.1016/j.bmcl.2016.09.008 BindingDB Entry DOI: 10.7270/Q2XP78MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||