 Found 411 hits with Last Name = 'irie' and Initial = 'h'
Found 411 hits with Last Name = 'irie' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA polymerase theta
(Homo sapiens) | BDBM50591018
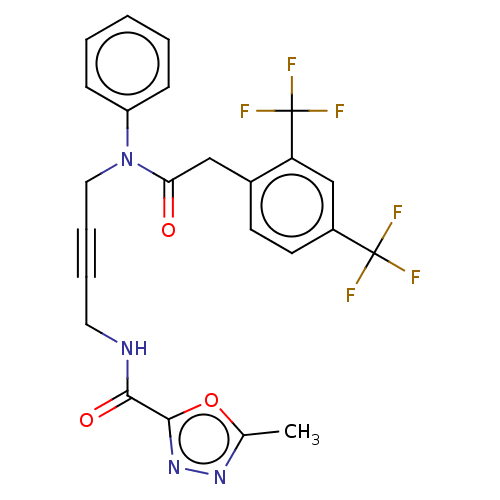
(CHEMBL5204203)Show SMILES Cc1nnc(o1)C(=O)NCC#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591016
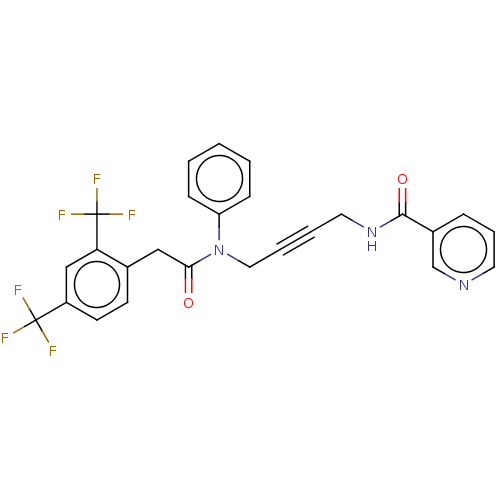
(CHEMBL5175156)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#CCNC(=O)c2cccnc2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591017
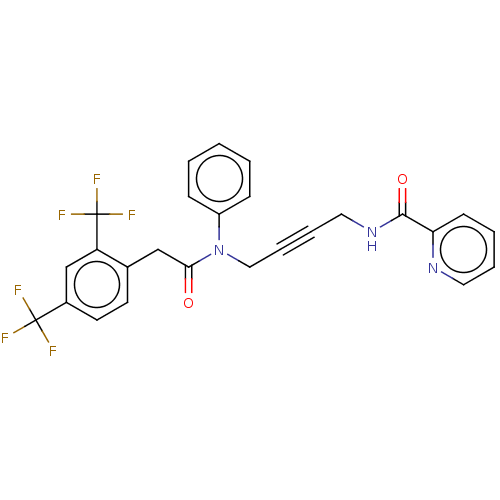
(CHEMBL5206022)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#CCNC(=O)c2ccccn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591022
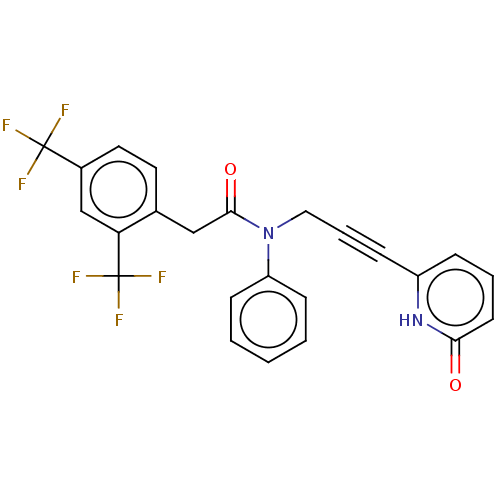
(CHEMBL5197367)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2cccc(=O)[nH]2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591035
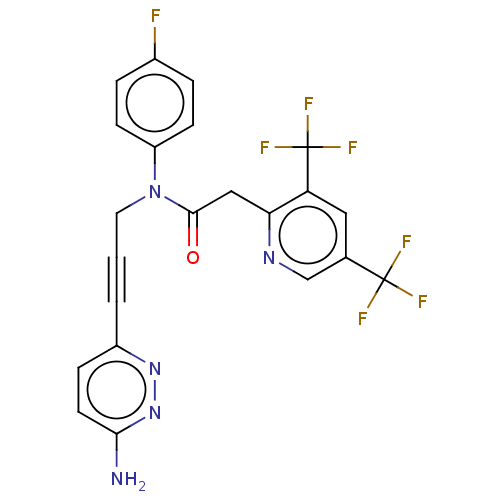
(CHEMBL5187422)Show SMILES Nc1ccc(nn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591030
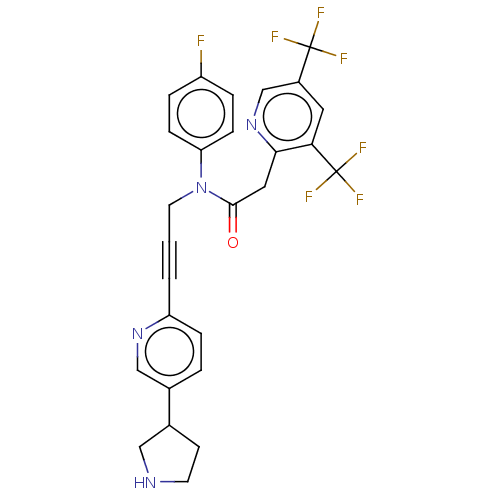
(CHEMBL5174509)Show SMILES Fc1ccc(cc1)N(CC#Cc1ccc(cn1)C1CCNC1)C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591026
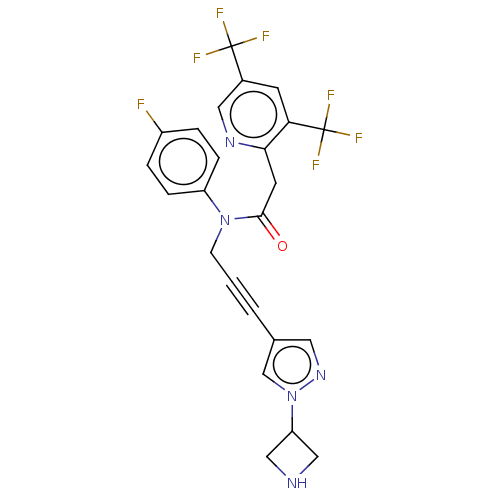
(CHEMBL5197173)Show SMILES Fc1ccc(cc1)N(CC#Cc1cnn(c1)C1CNC1)C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591015
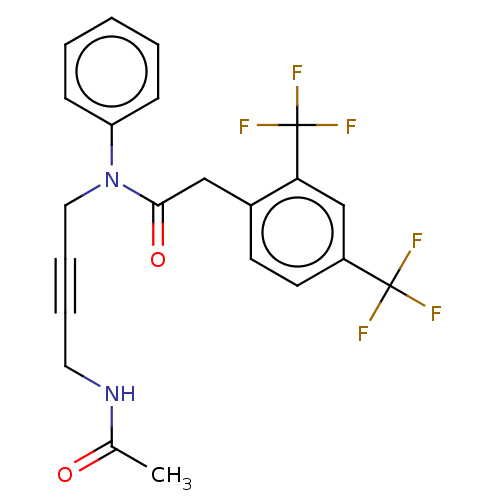
(CHEMBL5170373)Show SMILES CC(=O)NCC#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591028
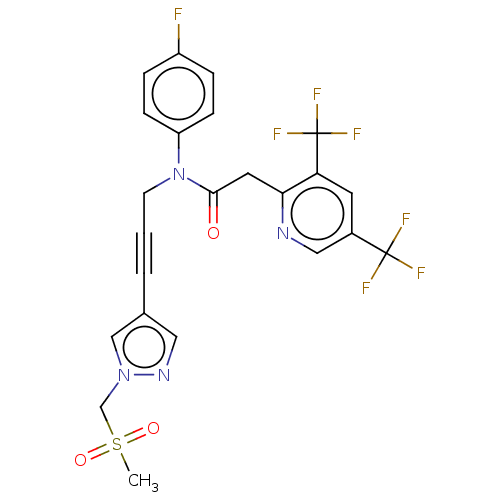
(CHEMBL5194637)Show SMILES CS(=O)(=O)Cn1cc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591027
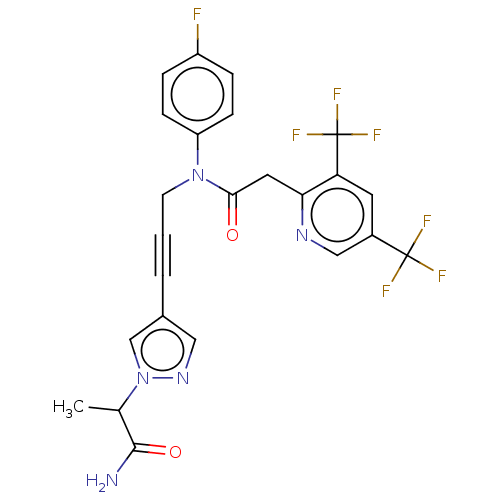
(CHEMBL5197021)Show SMILES CC(C(N)=O)n1cc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591019
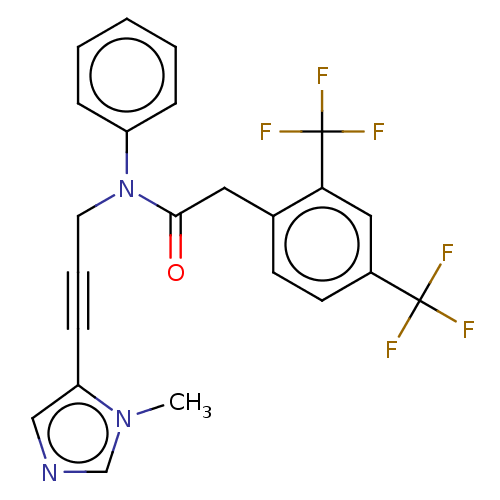
(CHEMBL5187337)Show SMILES Cn1cncc1C#CCN(C(=O)Cc1ccc(cc1C(F)(F)F)C(F)(F)F)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591024

(CHEMBL5188048)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2cccnn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
DNA polymerase theta
(Homo sapiens) | BDBM50591033
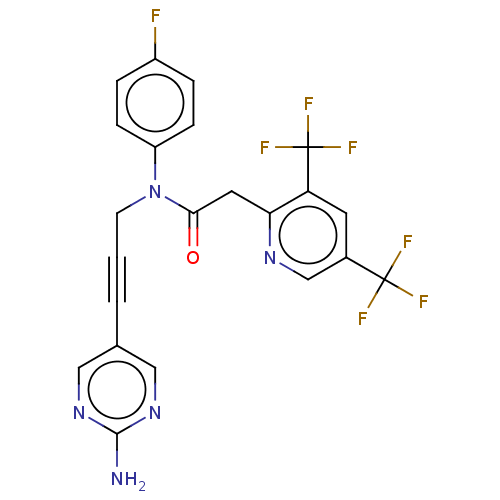
(CHEMBL5181369)Show SMILES Nc1ncc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591029
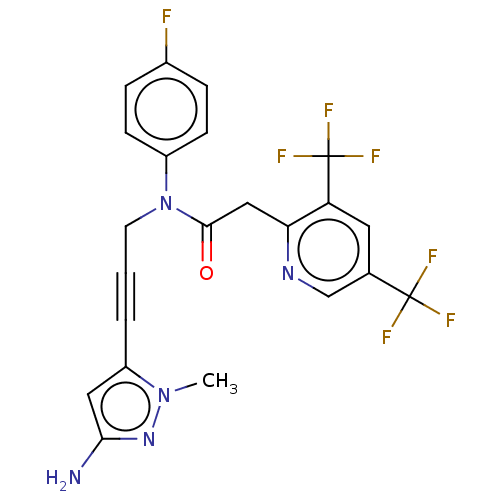
(CHEMBL5174376)Show SMILES Cn1nc(N)cc1C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591032
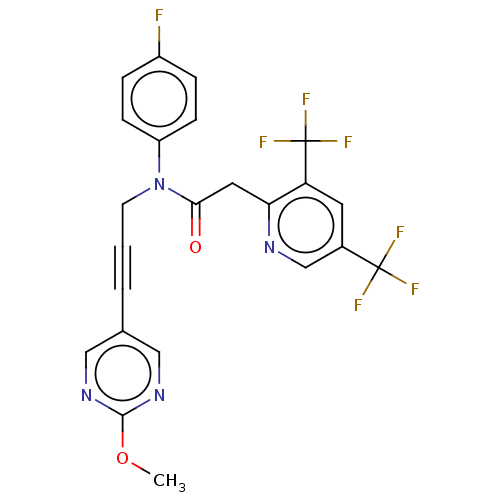
(CHEMBL5200250)Show SMILES COc1ncc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591021
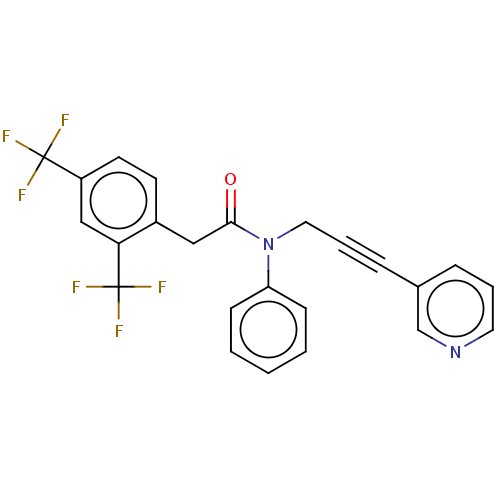
(CHEMBL5174283)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2cccnc2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376962
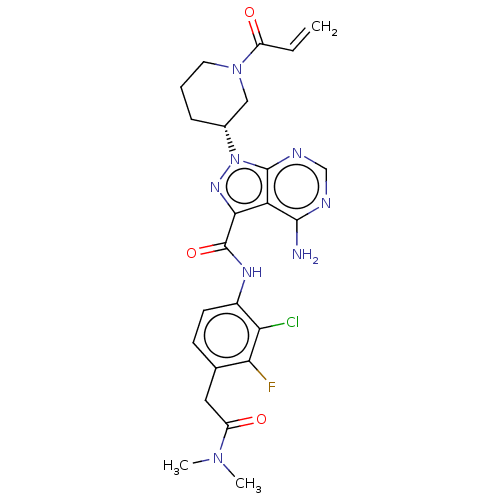
(US10329300, Example 50 | US11696917, Example 50 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1F Show InChI InChI=1S/C24H26ClFN8O3/c1-4-16(35)33-9-5-6-14(11-33)34-23-18(22(27)28-12-29-23)21(31-34)24(37)30-15-8-7-13(20(26)19(15)25)10-17(36)32(2)3/h4,7-8,12,14H,1,5-6,9-11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376962

(US10329300, Example 50 | US11696917, Example 50 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1F Show InChI InChI=1S/C24H26ClFN8O3/c1-4-16(35)33-9-5-6-14(11-33)34-23-18(22(27)28-12-29-23)21(31-34)24(37)30-15-8-7-13(20(26)19(15)25)10-17(36)32(2)3/h4,7-8,12,14H,1,5-6,9-11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376962

(US10329300, Example 50 | US11696917, Example 50 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1F Show InChI InChI=1S/C24H26ClFN8O3/c1-4-16(35)33-9-5-6-14(11-33)34-23-18(22(27)28-12-29-23)21(31-34)24(37)30-15-8-7-13(20(26)19(15)25)10-17(36)32(2)3/h4,7-8,12,14H,1,5-6,9-11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591020
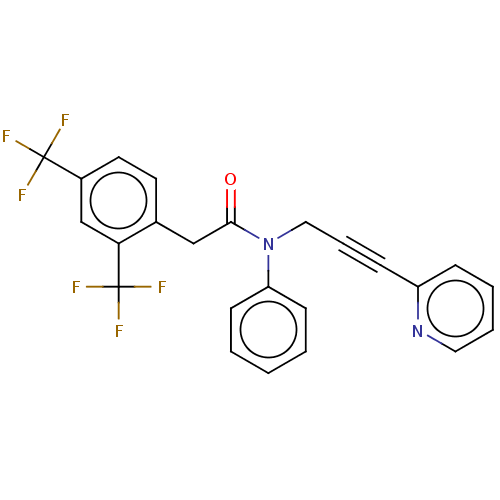
(CHEMBL5195161)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2ccccn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591023
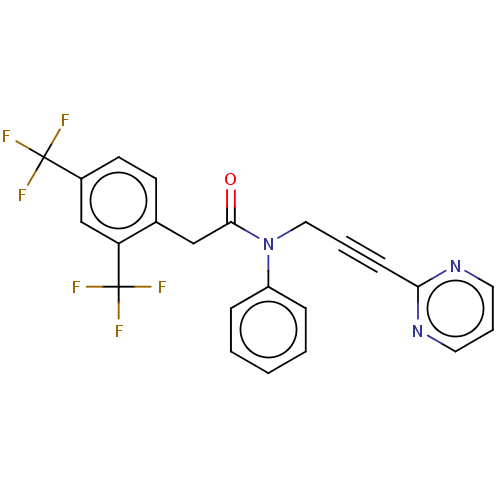
(CHEMBL5194194)Show SMILES FC(F)(F)c1ccc(CC(=O)N(CC#Cc2ncccn2)c2ccccc2)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376933
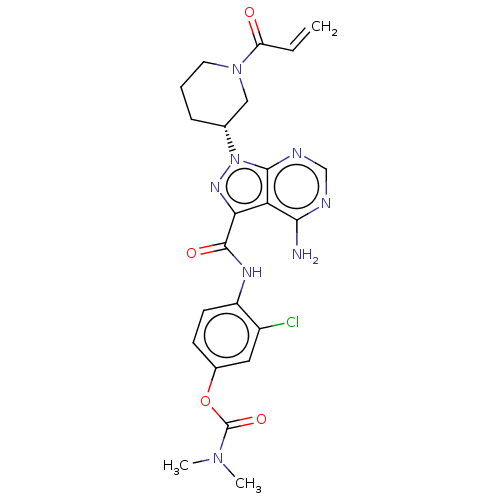
(US10329300, Example 27 | US11696917, Example 27 | ...)Show SMILES CN(C)C(=O)Oc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C23H25ClN8O4/c1-4-17(33)31-9-5-6-13(11-31)32-21-18(20(25)26-12-27-21)19(29-32)22(34)28-16-8-7-14(10-15(16)24)36-23(35)30(2)3/h4,7-8,10,12-13H,1,5-6,9,11H2,2-3H3,(H,28,34)(H2,25,26,27)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376933

(US10329300, Example 27 | US11696917, Example 27 | ...)Show SMILES CN(C)C(=O)Oc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C23H25ClN8O4/c1-4-17(33)31-9-5-6-13(11-31)32-21-18(20(25)26-12-27-21)19(29-32)22(34)28-16-8-7-14(10-15(16)24)36-23(35)30(2)3/h4,7-8,10,12-13H,1,5-6,9,11H2,2-3H3,(H,28,34)(H2,25,26,27)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376933

(US10329300, Example 27 | US11696917, Example 27 | ...)Show SMILES CN(C)C(=O)Oc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C23H25ClN8O4/c1-4-17(33)31-9-5-6-13(11-31)32-21-18(20(25)26-12-27-21)19(29-32)22(34)28-16-8-7-14(10-15(16)24)36-23(35)30(2)3/h4,7-8,10,12-13H,1,5-6,9,11H2,2-3H3,(H,28,34)(H2,25,26,27)/t13-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376912

(US10329300, Example 6 | US11696917, Example 6 | US...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C24H27ClN8O3/c1-4-18(34)32-9-5-6-15(12-32)33-23-20(22(26)27-13-28-23)21(30-33)24(36)29-17-8-7-14(10-16(17)25)11-19(35)31(2)3/h4,7-8,10,13,15H,1,5-6,9,11-12H2,2-3H3,(H,29,36)(H2,26,27,28)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376912

(US10329300, Example 6 | US11696917, Example 6 | US...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C24H27ClN8O3/c1-4-18(34)32-9-5-6-15(12-32)33-23-20(22(26)27-13-28-23)21(30-33)24(36)29-17-8-7-14(10-16(17)25)11-19(35)31(2)3/h4,7-8,10,13,15H,1,5-6,9,11-12H2,2-3H3,(H,29,36)(H2,26,27,28)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376912

(US10329300, Example 6 | US11696917, Example 6 | US...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C24H27ClN8O3/c1-4-18(34)32-9-5-6-15(12-32)33-23-20(22(26)27-13-28-23)21(30-33)24(36)29-17-8-7-14(10-16(17)25)11-19(35)31(2)3/h4,7-8,10,13,15H,1,5-6,9,11-12H2,2-3H3,(H,29,36)(H2,26,27,28)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591034
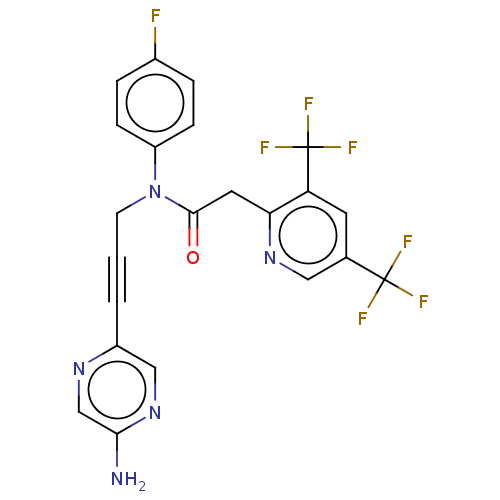
(CHEMBL5206029)Show SMILES Nc1cnc(cn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376924
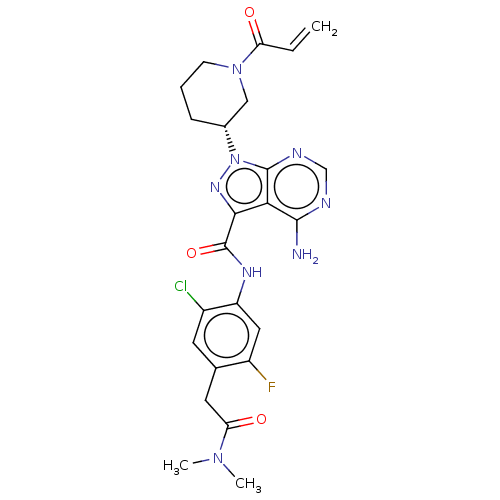
(US10329300, Example 18 | US11696917, Example 18 | ...)Show SMILES CN(C)C(=O)Cc1cc(Cl)c(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)cc1F Show InChI InChI=1S/C24H26ClFN8O3/c1-4-18(35)33-7-5-6-14(11-33)34-23-20(22(27)28-12-29-23)21(31-34)24(37)30-17-10-16(26)13(8-15(17)25)9-19(36)32(2)3/h4,8,10,12,14H,1,5-7,9,11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376924

(US10329300, Example 18 | US11696917, Example 18 | ...)Show SMILES CN(C)C(=O)Cc1cc(Cl)c(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)cc1F Show InChI InChI=1S/C24H26ClFN8O3/c1-4-18(35)33-7-5-6-14(11-33)34-23-20(22(27)28-12-29-23)21(31-34)24(37)30-17-10-16(26)13(8-15(17)25)9-19(36)32(2)3/h4,8,10,12,14H,1,5-7,9,11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376924

(US10329300, Example 18 | US11696917, Example 18 | ...)Show SMILES CN(C)C(=O)Cc1cc(Cl)c(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)cc1F Show InChI InChI=1S/C24H26ClFN8O3/c1-4-18(35)33-7-5-6-14(11-33)34-23-20(22(27)28-12-29-23)21(31-34)24(37)30-17-10-16(26)13(8-15(17)25)9-19(36)32(2)3/h4,8,10,12,14H,1,5-7,9,11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376920
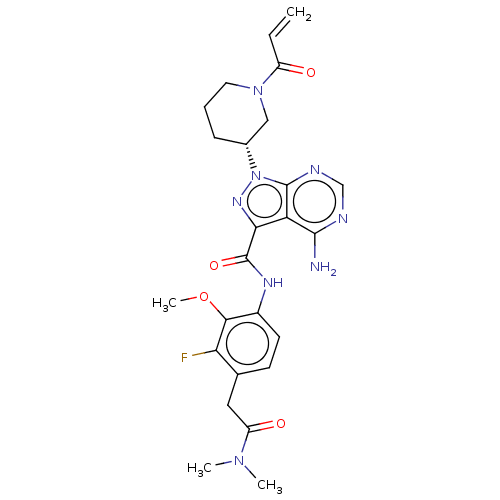
(US10329300, Example 14 | US11696917, Example 14 | ...)Show SMILES COc1c(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)ccc(CC(=O)N(C)C)c1F Show InChI InChI=1S/C25H29FN8O4/c1-5-17(35)33-10-6-7-15(12-33)34-24-19(23(27)28-13-29-24)21(31-34)25(37)30-16-9-8-14(11-18(36)32(2)3)20(26)22(16)38-4/h5,8-9,13,15H,1,6-7,10-12H2,2-4H3,(H,30,37)(H2,27,28,29)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376920

(US10329300, Example 14 | US11696917, Example 14 | ...)Show SMILES COc1c(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)ccc(CC(=O)N(C)C)c1F Show InChI InChI=1S/C25H29FN8O4/c1-5-17(35)33-10-6-7-15(12-33)34-24-19(23(27)28-13-29-24)21(31-34)25(37)30-16-9-8-14(11-18(36)32(2)3)20(26)22(16)38-4/h5,8-9,13,15H,1,6-7,10-12H2,2-4H3,(H,30,37)(H2,27,28,29)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376920

(US10329300, Example 14 | US11696917, Example 14 | ...)Show SMILES COc1c(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)ccc(CC(=O)N(C)C)c1F Show InChI InChI=1S/C25H29FN8O4/c1-5-17(35)33-10-6-7-15(12-33)34-24-19(23(27)28-13-29-24)21(31-34)25(37)30-16-9-8-14(11-18(36)32(2)3)20(26)22(16)38-4/h5,8-9,13,15H,1,6-7,10-12H2,2-4H3,(H,30,37)(H2,27,28,29)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591031
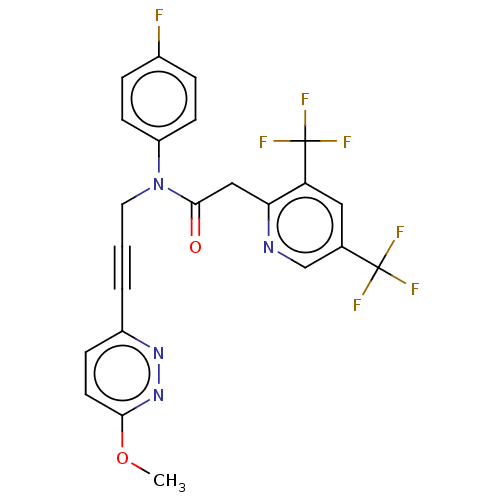
(CHEMBL5202450)Show SMILES COc1ccc(nn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376988
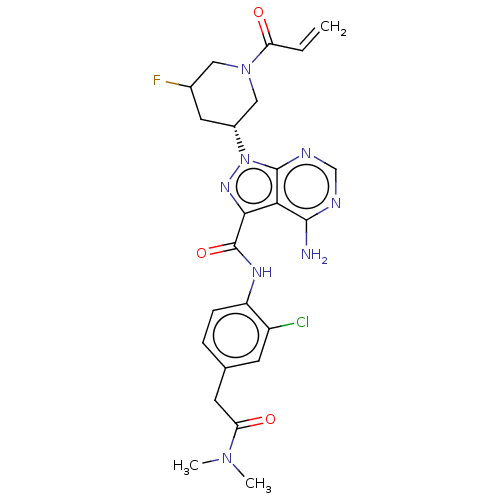
(US10329300, Example 76 | US11696917, Example 76 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CC(F)CN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C24H26ClFN8O3/c1-4-18(35)33-10-14(26)9-15(11-33)34-23-20(22(27)28-12-29-23)21(31-34)24(37)30-17-6-5-13(7-16(17)25)8-19(36)32(2)3/h4-7,12,14-15H,1,8-11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14?,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376988

(US10329300, Example 76 | US11696917, Example 76 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CC(F)CN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C24H26ClFN8O3/c1-4-18(35)33-10-14(26)9-15(11-33)34-23-20(22(27)28-12-29-23)21(31-34)24(37)30-17-6-5-13(7-16(17)25)8-19(36)32(2)3/h4-7,12,14-15H,1,8-11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14?,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376988

(US10329300, Example 76 | US11696917, Example 76 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CC(F)CN(C3)C(=O)C=C)c3ncnc(N)c23)c(Cl)c1 Show InChI InChI=1S/C24H26ClFN8O3/c1-4-18(35)33-10-14(26)9-15(11-33)34-23-20(22(27)28-12-29-23)21(31-34)24(37)30-17-6-5-13(7-16(17)25)8-19(36)32(2)3/h4-7,12,14-15H,1,8-11H2,2-3H3,(H,30,37)(H2,27,28,29)/t14?,15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376948
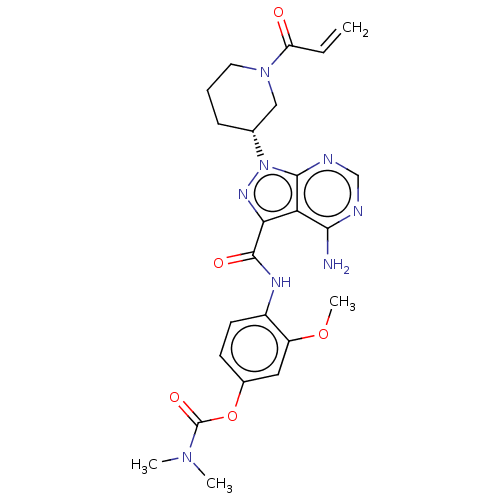
(US10329300, Example 36 | US11696917, Example 36 | ...)Show SMILES COc1cc(OC(=O)N(C)C)ccc1NC(=O)c1nn([C@@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 Show InChI InChI=1S/C24H28N8O5/c1-5-18(33)31-10-6-7-14(12-31)32-22-19(21(25)26-13-27-22)20(29-32)23(34)28-16-9-8-15(11-17(16)36-4)37-24(35)30(2)3/h5,8-9,11,13-14H,1,6-7,10,12H2,2-4H3,(H,28,34)(H2,25,26,27)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376948

(US10329300, Example 36 | US11696917, Example 36 | ...)Show SMILES COc1cc(OC(=O)N(C)C)ccc1NC(=O)c1nn([C@@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 Show InChI InChI=1S/C24H28N8O5/c1-5-18(33)31-10-6-7-14(12-31)32-22-19(21(25)26-13-27-22)20(29-32)23(34)28-16-9-8-15(11-17(16)36-4)37-24(35)30(2)3/h5,8-9,11,13-14H,1,6-7,10,12H2,2-4H3,(H,28,34)(H2,25,26,27)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376948

(US10329300, Example 36 | US11696917, Example 36 | ...)Show SMILES COc1cc(OC(=O)N(C)C)ccc1NC(=O)c1nn([C@@H]2CCCN(C2)C(=O)C=C)c2ncnc(N)c12 Show InChI InChI=1S/C24H28N8O5/c1-5-18(33)31-10-6-7-14(12-31)32-22-19(21(25)26-13-27-22)20(29-32)23(34)28-16-9-8-15(11-17(16)36-4)37-24(35)30(2)3/h5,8-9,11,13-14H,1,6-7,10,12H2,2-4H3,(H,28,34)(H2,25,26,27)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
DNA polymerase theta
(Homo sapiens) | BDBM50591035

(CHEMBL5187422)Show SMILES Nc1ccc(nn1)C#CCN(C(=O)Cc1ncc(cc1C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00998
BindingDB Entry DOI: 10.7270/Q2C53QVS |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376923
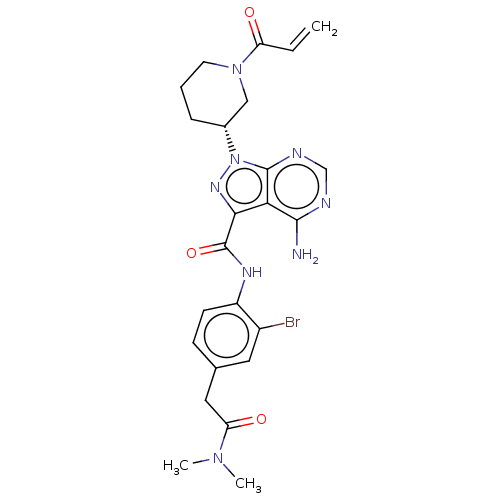
(US10329300, Example 17 | US11696917, Example 17 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Br)c1 Show InChI InChI=1S/C24H27BrN8O3/c1-4-18(34)32-9-5-6-15(12-32)33-23-20(22(26)27-13-28-23)21(30-33)24(36)29-17-8-7-14(10-16(17)25)11-19(35)31(2)3/h4,7-8,10,13,15H,1,5-6,9,11-12H2,2-3H3,(H,29,36)(H2,26,27,28)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376923

(US10329300, Example 17 | US11696917, Example 17 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Br)c1 Show InChI InChI=1S/C24H27BrN8O3/c1-4-18(34)32-9-5-6-15(12-32)33-23-20(22(26)27-13-28-23)21(30-33)24(36)29-17-8-7-14(10-16(17)25)11-19(35)31(2)3/h4,7-8,10,13,15H,1,5-6,9,11-12H2,2-3H3,(H,29,36)(H2,26,27,28)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376923

(US10329300, Example 17 | US11696917, Example 17 | ...)Show SMILES CN(C)C(=O)Cc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c(Br)c1 Show InChI InChI=1S/C24H27BrN8O3/c1-4-18(34)32-9-5-6-15(12-32)33-23-20(22(26)27-13-28-23)21(30-33)24(36)29-17-8-7-14(10-16(17)25)11-19(35)31(2)3/h4,7-8,10,13,15H,1,5-6,9,11-12H2,2-3H3,(H,29,36)(H2,26,27,28)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376945
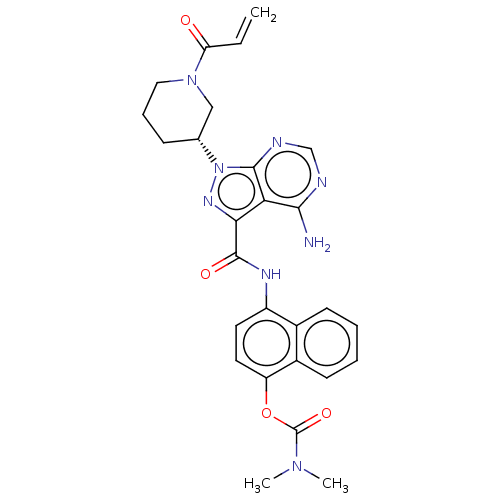
((R)-4-(1-(1-acryloylpiperidin-3-yl)-4-amino-1H-pyr...)Show SMILES CN(C)C(=O)Oc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c2ccccc12 Show InChI InChI=1S/C27H28N8O4/c1-4-21(36)34-13-7-8-16(14-34)35-25-22(24(28)29-15-30-25)23(32-35)26(37)31-19-11-12-20(39-27(38)33(2)3)18-10-6-5-9-17(18)19/h4-6,9-12,15-16H,1,7-8,13-14H2,2-3H3,(H,31,37)(H2,28,29,30)/t16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376945

((R)-4-(1-(1-acryloylpiperidin-3-yl)-4-amino-1H-pyr...)Show SMILES CN(C)C(=O)Oc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c2ccccc12 Show InChI InChI=1S/C27H28N8O4/c1-4-21(36)34-13-7-8-16(14-34)35-25-22(24(28)29-15-30-25)23(32-35)26(37)31-19-11-12-20(39-27(38)33(2)3)18-10-6-5-9-17(18)19/h4-6,9-12,15-16H,1,7-8,13-14H2,2-3H3,(H,31,37)(H2,28,29,30)/t16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite Paris 7-Denis Diderot
| Assay Description
For the inhibitory activity measurement of each compound, the compound of the present invention or staurosporine was first serially diluted with dime... |
Bioorg Med Chem 16: 1242-53 (2008)
BindingDB Entry DOI: 10.7270/Q2BR8VHD |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376945

((R)-4-(1-(1-acryloylpiperidin-3-yl)-4-amino-1H-pyr...)Show SMILES CN(C)C(=O)Oc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)c2ccccc12 Show InChI InChI=1S/C27H28N8O4/c1-4-21(36)34-13-7-8-16(14-34)35-25-22(24(28)29-15-30-25)23(32-35)26(37)31-19-11-12-20(39-27(38)33(2)3)18-10-6-5-9-17(18)19/h4-6,9-12,15-16H,1,7-8,13-14H2,2-3H3,(H,31,37)(H2,28,29,30)/t16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376941
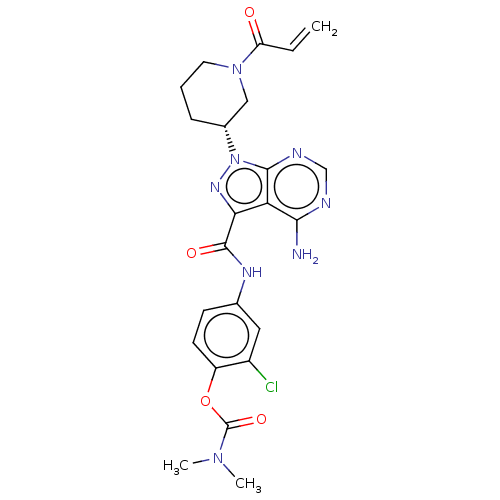
(US10329300, Example 29 | US11696917, Example 29 | ...)Show SMILES CN(C)C(=O)Oc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)cc1Cl Show InChI InChI=1S/C23H25ClN8O4/c1-4-17(33)31-9-5-6-14(11-31)32-21-18(20(25)26-12-27-21)19(29-32)22(34)28-13-7-8-16(15(24)10-13)36-23(35)30(2)3/h4,7-8,10,12,14H,1,5-6,9,11H2,2-3H3,(H,28,34)(H2,25,26,27)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2F193VV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM376941

(US10329300, Example 29 | US11696917, Example 29 | ...)Show SMILES CN(C)C(=O)Oc1ccc(NC(=O)c2nn([C@@H]3CCCN(C3)C(=O)C=C)c3ncnc(N)c23)cc1Cl Show InChI InChI=1S/C23H25ClN8O4/c1-4-17(33)31-9-5-6-14(11-31)32-21-18(20(25)26-12-27-21)19(29-32)22(34)28-13-7-8-16(15(24)10-13)36-23(35)30(2)3/h4,7-8,10,12,14H,1,5-6,9,11H2,2-3H3,(H,28,34)(H2,25,26,27)/t14-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TAIHO PHARMACEUTICAL CO., LTD.
US Patent
| Assay Description
For setting the conditions for the method for measuring the in vitro inhibitory activity of a compound against HER2-phosphorylating activity, Profile... |
US Patent US10329300 (2019)
BindingDB Entry DOI: 10.7270/Q2MG7RWF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data