 Found 102 hits with Last Name = 'konno' and Initial = 'h'
Found 102 hits with Last Name = 'konno' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Relative binding affinity for human estrogen receptor alpha by displacement of [3H]-estradiol |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50410843

(CHEMBL5281221)Show SMILES CNc1cccc(NC(=O)CN2N=C(c3ccccn3)c3ccccc3N(CC(=O)N3CCCC3)C2=O)c1 |t:12| Show InChI InChI=1S/C28H29N7O3/c1-29-20-9-8-10-21(17-20)31-25(36)18-35-28(38)34(19-26(37)33-15-6-7-16-33)24-13-3-2-11-22(24)27(32-35)23-12-4-5-14-30-23/h2-5,8-14,17,29H,6-7,15-16,18-19H2,1H3,(H,31,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity against melatonin receptor was determined |
Citation and Details
|
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 1
(Homo sapiens (Human)) | BDBM50573217
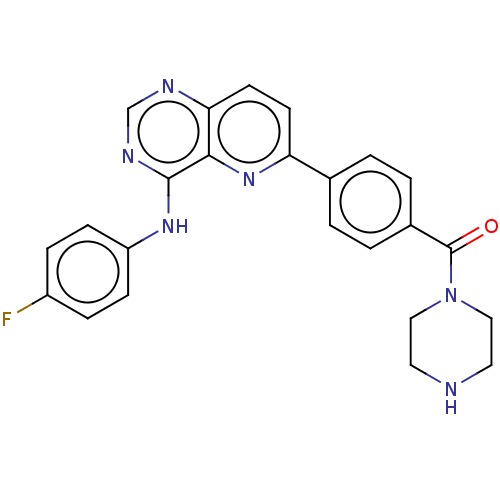
(CHEMBL4876307)Show SMILES Fc1ccc(Nc2ncnc3ccc(nc23)-c2ccc(cc2)C(=O)N2CCNCC2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50410844
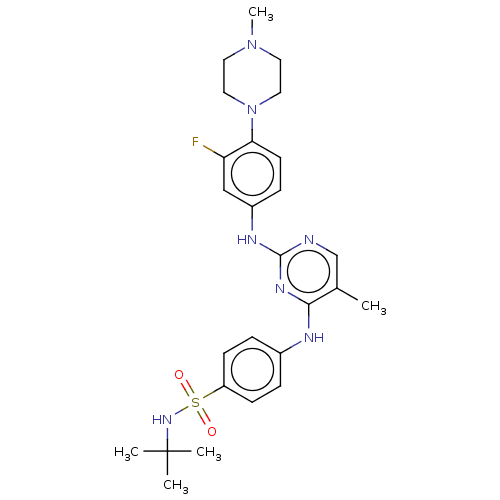
(CHEMBL5289028)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-n2ccnc2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C31H36N6O3/c1-31(2,3)27(38)19-36-26-15-8-7-14-25(26)29(22-10-5-4-6-11-22)34-37(30(36)40)20-28(39)33-23-12-9-13-24(18-23)35-17-16-32-21-35/h7-9,12-18,21-22H,4-6,10-11,19-20H2,1-3H3,(H,33,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration required to inhibit the HIV-1 recombinant Reverse Transcriptase (rRT) activity by 50%. Activated calf thymus DNA was used as template p... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50410844

(CHEMBL5289028)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-n2ccnc2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C31H36N6O3/c1-31(2,3)27(38)19-36-26-15-8-7-14-25(26)29(22-10-5-4-6-11-22)34-37(30(36)40)20-28(39)33-23-12-9-13-24(18-23)35-17-16-32-21-35/h7-9,12-18,21-22H,4-6,10-11,19-20H2,1-3H3,(H,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration required to inhibit the HIV-1 recombinant Reverse Transcriptase (rRT) activity by 50%. Activated calf thymus DNA was used as template p... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50410803
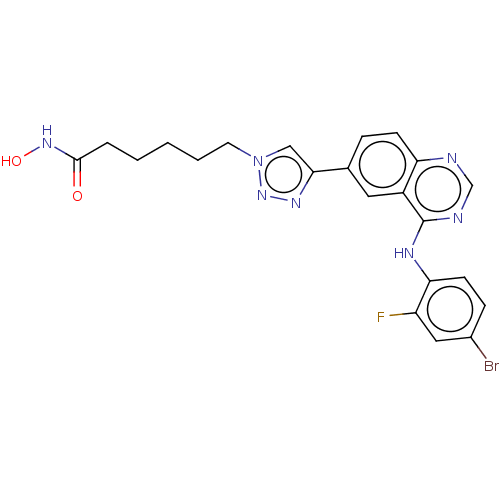
(CHEMBL5280359)Show SMILES FC(F)(F)c1cc(cc(c1)C(F)(F)F)C(=O)N1CCN(C\C(=N/OCCN2CCOCC2)c2ccccc2)C[C@H]1Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C36H37F6N5O3/c37-35(38,39)28-18-26(19-29(21-28)36(40,41)42)34(48)47-11-10-46(23-30(47)20-27-22-43-32-9-5-4-8-31(27)32)24-33(25-6-2-1-3-7-25)44-50-17-14-45-12-15-49-16-13-45/h1-9,18-19,21-22,30,43H,10-17,20,23-24H2/b44-33+/t30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat GnRH receptor assessed as luteinizing hormone release in rat pituitary cells |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50410843

(CHEMBL5281221)Show SMILES CNc1cccc(NC(=O)CN2N=C(c3ccccn3)c3ccccc3N(CC(=O)N3CCCC3)C2=O)c1 |t:12| Show InChI InChI=1S/C28H29N7O3/c1-29-20-9-8-10-21(17-20)31-25(36)18-35-28(38)34(19-26(37)33-15-6-7-16-33)24-13-3-2-11-22(24)27(32-35)23-12-4-5-14-30-23/h2-5,8-14,17,29H,6-7,15-16,18-19H2,1H3,(H,31,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Concentration required to inhibit the HIV-1 recombinant Reverse Transcriptase (rRT) activity by 50%. Activated calf thymus DNA was used as template p... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50512074

(CHEMBL4575813)Show SMILES ONC(=O)CCCCCCn1cc(Nc2ncc(Cl)c(Nc3ccc(Cl)cc3)n2)cn1 Show InChI InChI=1S/C20H23Cl2N7O2/c21-14-6-8-15(9-7-14)25-19-17(22)12-23-20(27-19)26-16-11-24-29(13-16)10-4-2-1-3-5-18(30)28-31/h6-9,11-13,31H,1-5,10H2,(H,28,30)(H2,23,25,26,27) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50410836
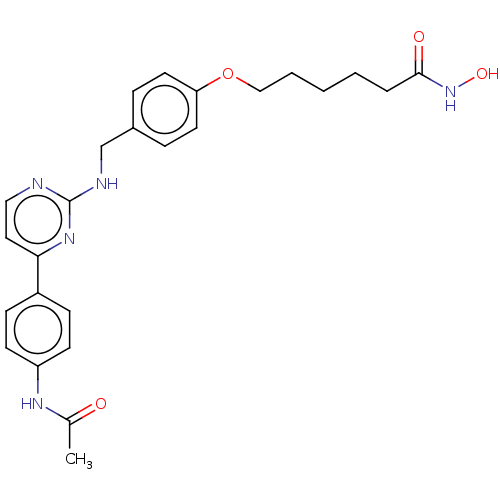
(CHEMBL5274303)Show SMILES CNc1cccc(NC(=O)CN2N=C(c3ccccn3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:12| Show InChI InChI=1S/C31H28N6O3/c1-21-10-3-4-13-24(21)28(38)19-36-27-16-6-5-14-25(27)30(26-15-7-8-17-33-26)35-37(31(36)40)20-29(39)34-23-12-9-11-22(18-23)32-2/h3-18,32H,19-20H2,1-2H3,(H,34,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50594749
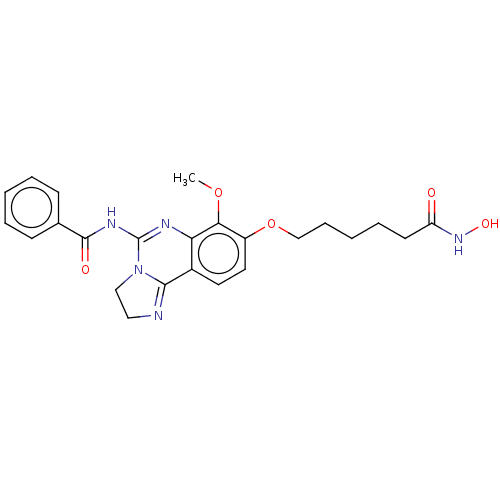
(CHEMBL5170300)Show SMILES COc1c(OCCCCCC(=O)NO)ccc2C3=NCCN3C(NC(=O)c3ccccc3)=Nc12 |c:33,t:17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50512074

(CHEMBL4575813)Show SMILES ONC(=O)CCCCCCn1cc(Nc2ncc(Cl)c(Nc3ccc(Cl)cc3)n2)cn1 Show InChI InChI=1S/C20H23Cl2N7O2/c21-14-6-8-15(9-7-14)25-19-17(22)12-23-20(27-19)26-16-11-24-29(13-16)10-4-2-1-3-5-18(30)28-31/h6-9,11-13,31H,1-5,10H2,(H,28,30)(H2,23,25,26,27) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50410803

(CHEMBL5280359)Show SMILES FC(F)(F)c1cc(cc(c1)C(F)(F)F)C(=O)N1CCN(C\C(=N/OCCN2CCOCC2)c2ccccc2)C[C@H]1Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C36H37F6N5O3/c37-35(38,39)28-18-26(19-29(21-28)36(40,41)42)34(48)47-11-10-46(23-30(47)20-27-22-43-32-9-5-4-8-31(27)32)24-33(25-6-2-1-3-7-25)44-50-17-14-45-12-15-49-16-13-45/h1-9,18-19,21-22,30,43H,10-17,20,23-24H2/b44-33+/t30-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50594749

(CHEMBL5170300)Show SMILES COc1c(OCCCCCC(=O)NO)ccc2C3=NCCN3C(NC(=O)c3ccccc3)=Nc12 |c:33,t:17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50410838
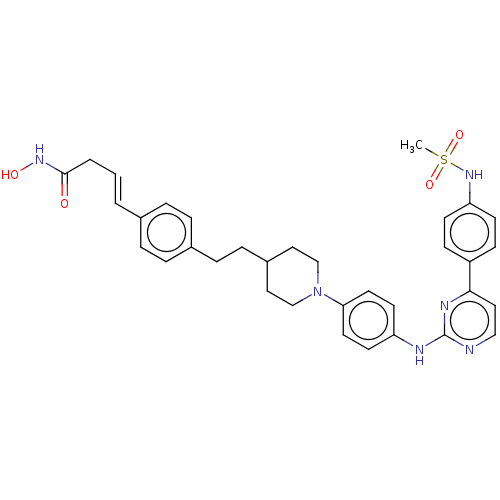
(CHEMBL5272716)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)N2CCOCC2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C32H41N5O4/c1-32(2,3)28(38)21-36-27-15-8-7-14-26(27)30(23-10-5-4-6-11-23)34-37(31(36)40)22-29(39)33-24-12-9-13-25(20-24)35-16-18-41-19-17-35/h7-9,12-15,20,23H,4-6,10-11,16-19,21-22H2,1-3H3,(H,33,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50410804
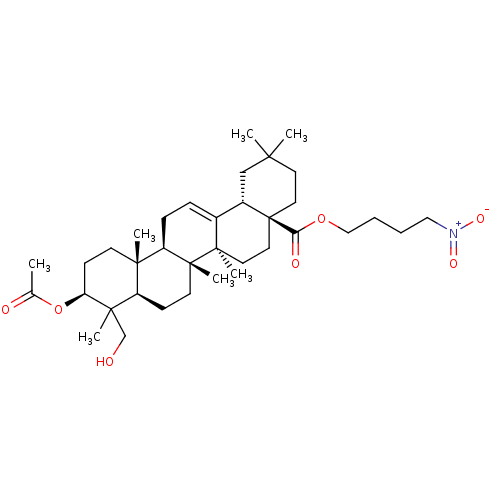
(CHEMBL5287477)Show SMILES FC(F)(F)c1cc(cc(c1)C(F)(F)F)C(=O)N1CCN(CC\C(=N/OCCN2CCOCC2)c2ccccc2)C[C@H]1Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C37H39F6N5O3/c38-36(39,40)29-20-27(21-30(23-29)37(41,42)43)35(49)48-13-12-47(25-31(48)22-28-24-44-34-9-5-4-8-32(28)34)11-10-33(26-6-2-1-3-7-26)45-51-19-16-46-14-17-50-18-15-46/h1-9,20-21,23-24,31,44H,10-19,22,25H2/b45-33+/t31-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50410803

(CHEMBL5280359)Show SMILES FC(F)(F)c1cc(cc(c1)C(F)(F)F)C(=O)N1CCN(C\C(=N/OCCN2CCOCC2)c2ccccc2)C[C@H]1Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C36H37F6N5O3/c37-35(38,39)28-18-26(19-29(21-28)36(40,41)42)34(48)47-11-10-46(23-30(47)20-27-22-43-32-9-5-4-8-31(27)32)24-33(25-6-2-1-3-7-25)44-50-17-14-45-12-15-49-16-13-45/h1-9,18-19,21-22,30,43H,10-17,20,23-24H2/b44-33+/t30-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50594749

(CHEMBL5170300)Show SMILES COc1c(OCCCCCC(=O)NO)ccc2C3=NCCN3C(NC(=O)c3ccccc3)=Nc12 |c:33,t:17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50410836

(CHEMBL5274303)Show SMILES CNc1cccc(NC(=O)CN2N=C(c3ccccn3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:12| Show InChI InChI=1S/C31H28N6O3/c1-21-10-3-4-13-24(21)28(38)19-36-27-16-6-5-14-25(27)30(26-15-7-8-17-33-26)35-37(31(36)40)20-29(39)34-23-12-9-11-22(18-23)32-2/h3-18,32H,19-20H2,1-2H3,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50410840
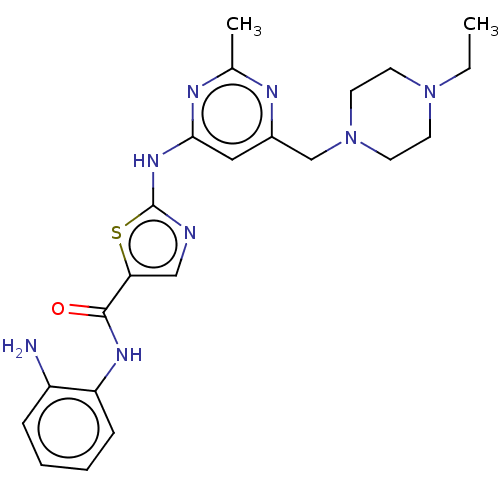
(CHEMBL5284623)Show SMILES CNCCN(C)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1 |t:16| Show InChI InChI=1S/C32H44N6O3/c1-32(2,3)28(39)21-37-27-17-10-9-16-26(27)30(23-12-7-6-8-13-23)35-38(31(37)41)22-29(40)34-24-14-11-15-25(20-24)36(5)19-18-33-4/h9-11,14-17,20,23,33H,6-8,12-13,18-19,21-22H2,1-5H3,(H,34,40) | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-beta
(Homo sapiens (Human)) | BDBM50410828

(CHEMBL5266387)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)[C@@]([#7])([#6]-[#8])[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C47H67N15O13S2/c48-35(65)15-14-29-39(69)59-32(20-36(49)66)42(72)60-33(44(74)62-17-5-9-34(62)43(73)57-28(8-4-16-54-46(51)52)38(68)55-21-37(50)67)22-76-77-24-47(53,23-63)45(75)61-31(19-26-10-12-27(64)13-11-26)41(71)58-30(40(70)56-29)18-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,63-64H,4-5,8-9,14-24,53H2,(H2,48,65)(H2,49,66)(H2,50,67)(H,55,68)(H,56,70)(H,57,73)(H,58,71)(H,59,69)(H,60,72)(H,61,75)(H4,51,52,54)/t28-,29-,30-,31-,32-,33+,34-,47+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity in phenoxybenzamine-treated rat by Pressor assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
MAP kinase-interacting serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50573217

(CHEMBL4876307)Show SMILES Fc1ccc(Nc2ncnc3ccc(nc23)-c2ccc(cc2)C(=O)N2CCNCC2)cc1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50410800
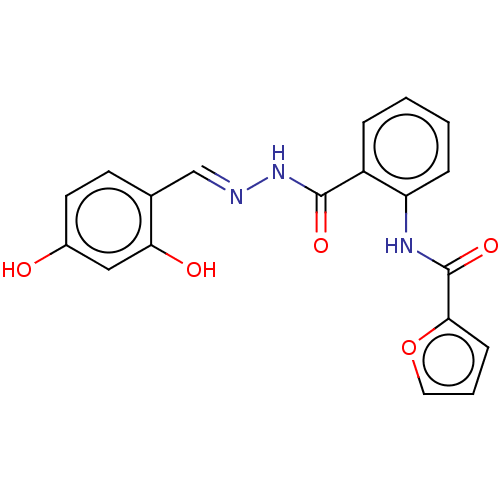
(CHEMBL5206193)Show SMILES CNCCO\N=C(\C)CN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H31F6N5O2/c1-18(37-41-10-7-35-2)16-38-8-9-39(23(17-38)13-20-15-36-25-6-4-3-5-24(20)25)26(40)19-11-21(27(29,30)31)14-22(12-19)28(32,33)34/h3-6,11-12,14-15,23,35-36H,7-10,13,16-17H2,1-2H3/b37-18-/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50410844

(CHEMBL5289028)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)-n2ccnc2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C31H36N6O3/c1-31(2,3)27(38)19-36-26-15-8-7-14-25(26)29(22-10-5-4-6-11-22)34-37(30(36)40)20-28(39)33-23-12-9-13-24(18-23)35-17-16-32-21-35/h7-9,12-18,21-22H,4-6,10-11,19-20H2,1-3H3,(H,33,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity against melatonin receptor was determined |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50410845
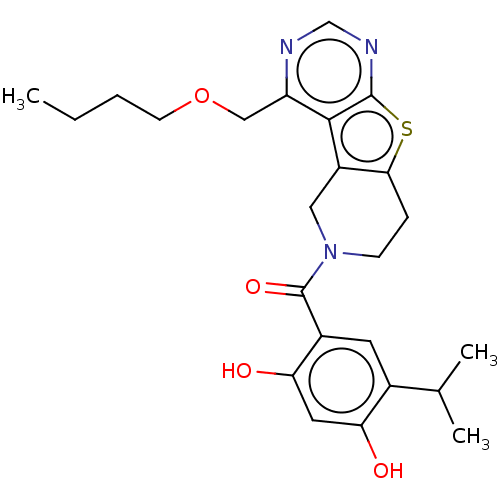
(CHEMBL5273021)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(CO)c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C29H36N4O4/c1-29(2,3)25(35)17-32-24-15-8-7-14-23(24)27(21-11-5-4-6-12-21)31-33(28(32)37)18-26(36)30-22-13-9-10-20(16-22)19-34/h7-10,13-16,21,34H,4-6,11-12,17-19H2,1-3H3,(H,30,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Relative binding affinity for sheep uterine estrogen receptor by displacement of [3H]estradiol |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50410828

(CHEMBL5266387)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)[C@@]([#7])([#6]-[#8])[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C47H67N15O13S2/c48-35(65)15-14-29-39(69)59-32(20-36(49)66)42(72)60-33(44(74)62-17-5-9-34(62)43(73)57-28(8-4-16-54-46(51)52)38(68)55-21-37(50)67)22-76-77-24-47(53,23-63)45(75)61-31(19-26-10-12-27(64)13-11-26)41(71)58-30(40(70)56-29)18-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,63-64H,4-5,8-9,14-24,53H2,(H2,48,65)(H2,49,66)(H2,50,67)(H,55,68)(H,56,70)(H,57,73)(H,58,71)(H,59,69)(H,60,72)(H,61,75)(H4,51,52,54)/t28-,29-,30-,31-,32-,33+,34-,47+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50410802
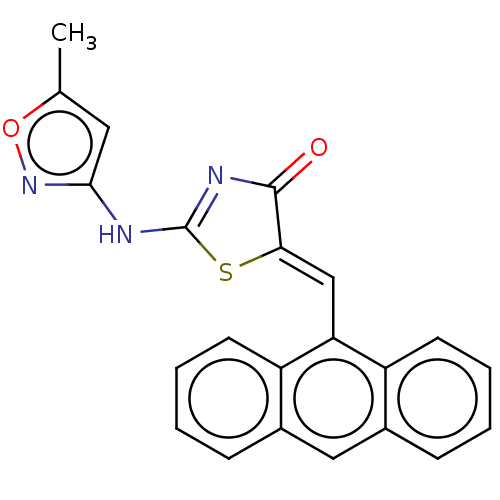
(CHEMBL5272853)Show SMILES C\C(CN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)=N\OCCCN1CCOCC1 Show InChI InChI=1S/C32H37F6N5O3/c1-22(40-46-12-4-7-41-10-13-45-14-11-41)20-42-8-9-43(27(21-42)17-24-19-39-29-6-3-2-5-28(24)29)30(44)23-15-25(31(33,34)35)18-26(16-23)32(36,37)38/h2-3,5-6,15-16,18-19,27,39H,4,7-14,17,20-21H2,1H3/b40-22-/t27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50410838

(CHEMBL5272716)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(c2)N2CCOCC2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C32H41N5O4/c1-32(2,3)28(38)21-36-27-15-8-7-14-26(27)30(23-10-5-4-6-11-23)34-37(31(36)40)22-29(39)33-24-12-9-13-25(20-24)35-16-18-41-19-17-35/h7-9,12-15,20,23H,4-6,10-11,16-19,21-22H2,1-3H3,(H,33,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50410845

(CHEMBL5273021)Show SMILES CC(C)(C)C(=O)CN1c2ccccc2C(=NN(CC(=O)Nc2cccc(CO)c2)C1=O)C1CCCCC1 |c:15| Show InChI InChI=1S/C29H36N4O4/c1-29(2,3)25(35)17-32-24-15-8-7-14-23(24)27(21-11-5-4-6-12-21)31-33(28(32)37)18-26(36)30-22-13-9-10-20(16-22)19-34/h7-10,13-16,21,34H,4-6,11-12,17-19H2,1-3H3,(H,30,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Relative binding affinity for human estrogen receptor beta by displacement of [3H]estradiol |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50594749

(CHEMBL5170300)Show SMILES COc1c(OCCCCCC(=O)NO)ccc2C3=NCCN3C(NC(=O)c3ccccc3)=Nc12 |c:33,t:17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50594749

(CHEMBL5170300)Show SMILES COc1c(OCCCCCC(=O)NO)ccc2C3=NCCN3C(NC(=O)c3ccccc3)=Nc12 |c:33,t:17| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50484478

(CHEMBL1929019 | jm5b01461, Compound 47)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1c[nH]cn1)C=O |r| Show InChI InChI=1S/C28H45N7O7/c1-16(2)24(35-25(39)17(3)31-27(41)23(14-37)32-18(4)38)28(42)34-22(10-19-8-6-5-7-9-19)26(40)33-21(13-36)11-20-12-29-15-30-20/h12-13,15-17,19,21-24,37H,5-11,14H2,1-4H3,(H,29,30)(H,31,41)(H,32,38)(H,33,40)(H,34,42)(H,35,39)/t17-,21-,22-,23-,24-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis |
J Med Chem 54: 7962-73 (2011)
Article DOI: 10.1021/jm200870n
BindingDB Entry DOI: 10.7270/Q29G5QNB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50410798

(CHEMBL5283543)Show SMILES COc1ccccc1CNCCNC(=O)N1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C33H33F6N5O3/c1-47-29-9-5-2-6-21(29)18-40-10-11-41-31(46)43-12-13-44(26(20-43)16-23-19-42-28-8-4-3-7-27(23)28)30(45)22-14-24(32(34,35)36)17-25(15-22)33(37,38)39/h2-9,14-15,17,19,26,40,42H,10-13,16,18,20H2,1H3,(H,41,46)/t26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50594716
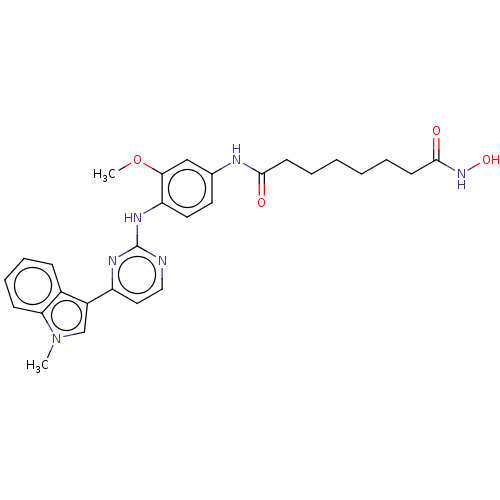
(CHEMBL5191956)Show SMILES COc1cc(NC(=O)CCCCCCC(=O)NO)ccc1Nc1nccc(n1)-c1cn(C)c2ccccc12 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50410841

(CHEMBL5266767)Show SMILES CN(C)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1 |t:13| Show InChI InChI=1S/C30H39N5O3/c1-30(2,3)26(36)19-34-25-17-10-9-16-24(25)28(21-12-7-6-8-13-21)32-35(29(34)38)20-27(37)31-22-14-11-15-23(18-22)33(4)5/h9-11,14-18,21H,6-8,12-13,19-20H2,1-5H3,(H,31,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to DC-SIGN extracellular domain (unknown origin) expressed in Escherichia coli after 1 hr by ELISA |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50410801
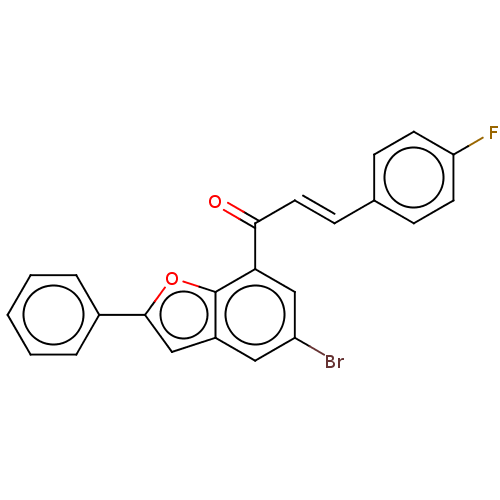
(CHEMBL5276251)Show SMILES C\C(CN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)=N\OCCN Show InChI InChI=1S/C27H29F6N5O2/c1-17(36-40-9-6-34)15-37-7-8-38(22(16-37)12-19-14-35-24-5-3-2-4-23(19)24)25(39)18-10-20(26(28,29)30)13-21(11-18)27(31,32)33/h2-5,10-11,13-14,22,35H,6-9,12,15-16,34H2,1H3/b36-17-/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50594719
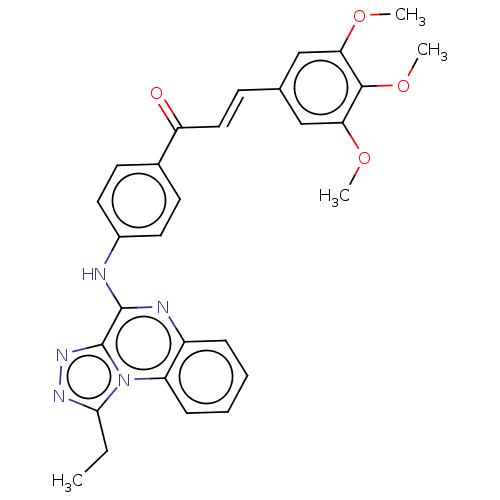
(CHEMBL5186583)Show SMILES CCc1nnc2c(Nc3ccc(cc3)C(=O)\C=C\c3cc(OC)c(OC)c(OC)c3)nc3ccccc3n12 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50484484
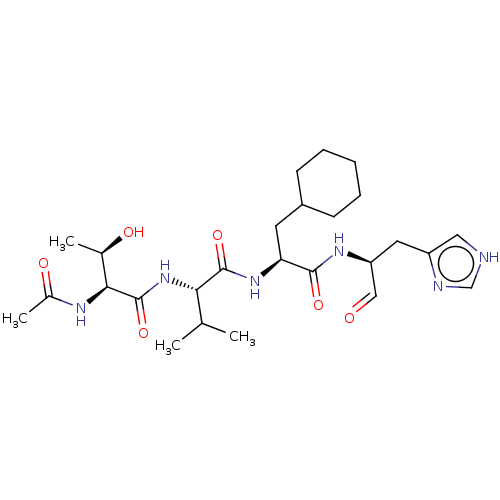
(CHEMBL1929023)Show SMILES CC(C)[C@H](NC(=O)[C@@H](NC(C)=O)[C@@H](C)O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1c[nH]cn1)C=O |r| Show InChI InChI=1S/C26H42N6O6/c1-15(2)22(32-26(38)23(16(3)34)29-17(4)35)25(37)31-21(10-18-8-6-5-7-9-18)24(36)30-20(13-33)11-19-12-27-14-28-19/h12-16,18,20-23,34H,5-11H2,1-4H3,(H,27,28)(H,29,35)(H,30,36)(H,31,37)(H,32,38)/t16-,20+,21+,22+,23+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis |
J Med Chem 54: 7962-73 (2011)
Article DOI: 10.1021/jm200870n
BindingDB Entry DOI: 10.7270/Q29G5QNB |
More data for this
Ligand-Target Pair | |
Tubulin beta-4B chain
(Homo sapiens) | BDBM50410800

(CHEMBL5206193)Show SMILES CNCCO\N=C(\C)CN1CCN([C@H](Cc2c[nH]c3ccccc23)C1)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H31F6N5O2/c1-18(37-41-10-7-35-2)16-38-8-9-39(23(17-38)13-20-15-36-25-6-4-3-5-24(20)25)26(40)19-11-21(27(29,30)31)14-22(12-19)28(32,33)34/h3-6,11-12,14-15,23,35-36H,7-10,13,16-17H2,1-2H3/b37-18-/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50410828

(CHEMBL5266387)Show SMILES [#7]-[#6](=O)-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)[C@@]([#7])([#6]-[#8])[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C47H67N15O13S2/c48-35(65)15-14-29-39(69)59-32(20-36(49)66)42(72)60-33(44(74)62-17-5-9-34(62)43(73)57-28(8-4-16-54-46(51)52)38(68)55-21-37(50)67)22-76-77-24-47(53,23-63)45(75)61-31(19-26-10-12-27(64)13-11-26)41(71)58-30(40(70)56-29)18-25-6-2-1-3-7-25/h1-3,6-7,10-13,28-34,63-64H,4-5,8-9,14-24,53H2,(H2,48,65)(H2,49,66)(H2,50,67)(H,55,68)(H,56,70)(H,57,73)(H,58,71)(H,59,69)(H,60,72)(H,61,75)(H4,51,52,54)/t28-,29-,30-,31-,32-,33+,34-,47+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain-containing protein 4
(Homo sapiens (Human)) | BDBM50410843

(CHEMBL5281221)Show SMILES CNc1cccc(NC(=O)CN2N=C(c3ccccn3)c3ccccc3N(CC(=O)N3CCCC3)C2=O)c1 |t:12| Show InChI InChI=1S/C28H29N7O3/c1-29-20-9-8-10-21(17-20)31-25(36)18-35-28(38)34(19-26(37)33-15-6-7-16-33)24-13-3-2-11-22(24)27(32-35)23-12-4-5-14-30-23/h2-5,8-14,17,29H,6-7,15-16,18-19H2,1H3,(H,31,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine as substrate measured for 1 min |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50410840

(CHEMBL5284623)Show SMILES CNCCN(C)c1cccc(NC(=O)CN2N=C(C3CCCCC3)c3ccccc3N(CC(=O)C(C)(C)C)C2=O)c1 |t:16| Show InChI InChI=1S/C32H44N6O3/c1-32(2,3)28(39)21-37-27-17-10-9-16-26(27)30(23-12-7-6-8-13-23)35-38(31(37)41)22-29(40)34-24-14-11-15-25(20-24)36(5)19-18-33-4/h9-11,14-17,20,23,33H,6-8,12-13,18-19,21-22H2,1-5H3,(H,34,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at M3 receptor in Dunkin-Hartley guinea pig trachea assessed as inhibition of methacholine-induced airway smooth muscle contracti... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50410830
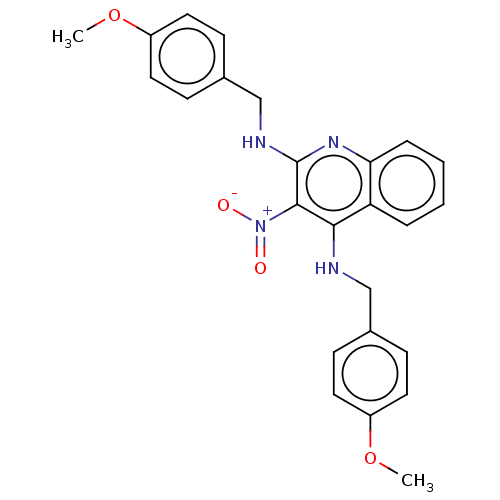
(CHEMBL5286373)Show SMILES [#7]-[#6@@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6]-1=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C52H69N15O12S2/c53-32-26-80-81-27-37(51(79)67-22-8-14-38(67)49(77)62-33(13-7-21-59-52(57)58)45(73)60-25-41(56)71)65-48(76)36(24-40(55)70)63-46(74)34(19-20-39(54)69)61-47(75)35(23-28-15-17-31(68)18-16-28)64-50(78)43(66-44(32)72)42(29-9-3-1-4-10-29)30-11-5-2-6-12-30/h1-6,9-12,15-18,32-38,42-43,68H,7-8,13-14,19-27,53H2,(H2,54,69)(H2,55,70)(H2,56,71)(H,60,73)(H,61,75)(H,62,77)(H,63,74)(H,64,78)(H,65,76)(H,66,72)(H4,57,58,59)/t32-,33+,34+,35+,36+,37-,38+,43+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50008923

((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccccc4cc3Cn1c2=O |r| Show InChI InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assay |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50484483
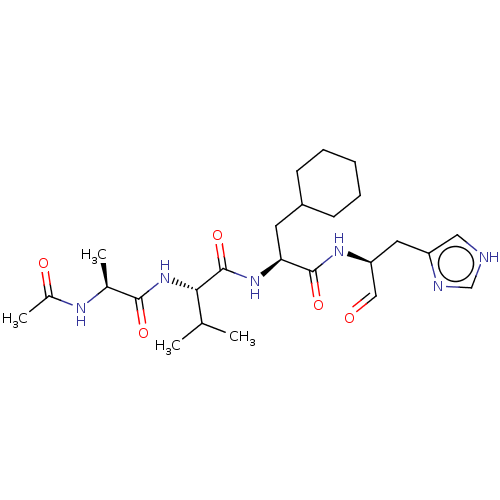
(CHEMBL1929020 | acs.jmedchem.1c00409_ST.132)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(C)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1c[nH]cn1)C=O |r| Show InChI InChI=1S/C25H40N6O5/c1-15(2)22(31-23(34)16(3)28-17(4)33)25(36)30-21(10-18-8-6-5-7-9-18)24(35)29-20(13-32)11-19-12-26-14-27-19/h12-16,18,20-22H,5-11H2,1-4H3,(H,26,27)(H,28,33)(H,29,35)(H,30,36)(H,31,34)/t16-,20-,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis |
J Med Chem 54: 7962-73 (2011)
Article DOI: 10.1021/jm200870n
BindingDB Entry DOI: 10.7270/Q29G5QNB |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50410829
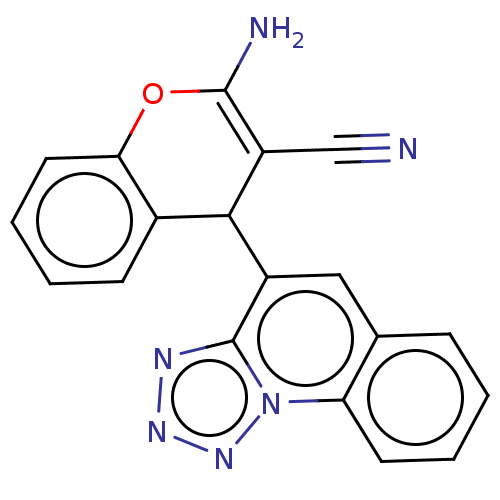
(CHEMBL5275527)Show SMILES CC(C)C[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H]1CSSC2(CCCCC2)CC(=O)N2CCN([C@@H](Cc3ccccc3)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1)C(=O)[C@@H]2Cc1ccccc1)C(=O)NCC(N)=O Show InChI InChI=1S/C53H73N11O11S2/c1-32(2)25-36(46(69)57-30-44(56)67)60-49(72)39-17-12-22-63(39)51(74)38-31-76-77-53(20-10-5-11-21-53)29-45(68)62-23-24-64(52(75)41(62)27-34-15-8-4-9-16-34)40(26-33-13-6-3-7-14-33)50(73)58-35(18-19-42(54)65)47(70)59-37(28-43(55)66)48(71)61-38/h3-4,6-9,13-16,32,35-41H,5,10-12,17-31H2,1-2H3,(H2,54,65)(H2,55,66)(H2,56,67)(H,57,69)(H,58,73)(H,59,70)(H,60,72)(H,61,71)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of P-glycoprotein expressed in MDCK-MDR1 cells by calcein AM assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50410836

(CHEMBL5274303)Show SMILES CNc1cccc(NC(=O)CN2N=C(c3ccccn3)c3ccccc3N(CC(=O)c3ccccc3C)C2=O)c1 |t:12| Show InChI InChI=1S/C31H28N6O3/c1-21-10-3-4-13-24(21)28(38)19-36-27-16-6-5-14-25(27)30(26-15-7-8-17-33-26)35-37(31(36)40)20-29(39)34-23-12-9-11-22(18-23)32-2/h3-18,32H,19-20H2,1-2H3,(H,34,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 306 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at 5HT3 receptor in spontaneously beating guinea pig right atrium assessed as inhibition of serotonin-induced maximum response by... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50484491
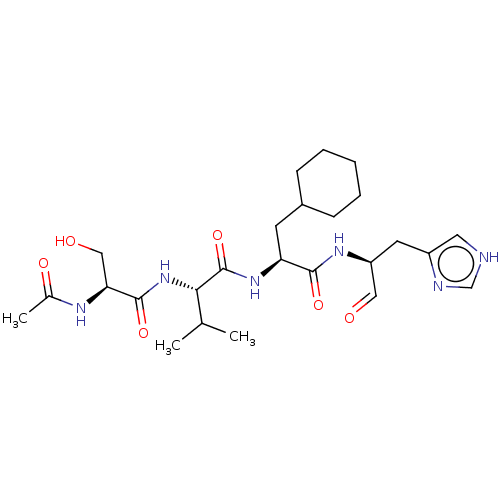
(CHEMBL1929022 | acs.jmedchem.1c00409_ST.144)Show SMILES CC(C)[C@H](NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1c[nH]cn1)C=O |r| Show InChI InChI=1S/C25H40N6O6/c1-15(2)22(31-24(36)21(13-33)28-16(3)34)25(37)30-20(9-17-7-5-4-6-8-17)23(35)29-19(12-32)10-18-11-26-14-27-18/h11-12,14-15,17,19-22,33H,4-10,13H2,1-3H3,(H,26,27)(H,28,34)(H,29,35)(H,30,37)(H,31,36)/t19-,20-,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis |
J Med Chem 54: 7962-73 (2011)
Article DOI: 10.1021/jm200870n
BindingDB Entry DOI: 10.7270/Q29G5QNB |
More data for this
Ligand-Target Pair | |
Chymotrypsin-like protease CTRL-1
(Homo sapiens (Human)) | BDBM50484487
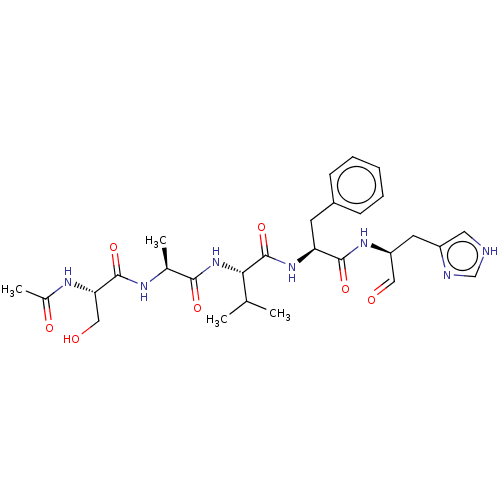
(CHEMBL1929018 | acs.jmedchem.1c00409_ST.150)Show SMILES CC(C)[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C=O |r| Show InChI InChI=1S/C28H39N7O7/c1-16(2)24(35-25(39)17(3)31-27(41)23(14-37)32-18(4)38)28(42)34-22(10-19-8-6-5-7-9-19)26(40)33-21(13-36)11-20-12-29-15-30-20/h5-9,12-13,15-17,21-24,37H,10-11,14H2,1-4H3,(H,29,30)(H,31,41)(H,32,38)(H,33,40)(H,34,42)(H,35,39)/t17-,21-,22-,23-,24-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis |
J Med Chem 54: 7962-73 (2011)
Article DOI: 10.1021/jm200870n
BindingDB Entry DOI: 10.7270/Q29G5QNB |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50601447
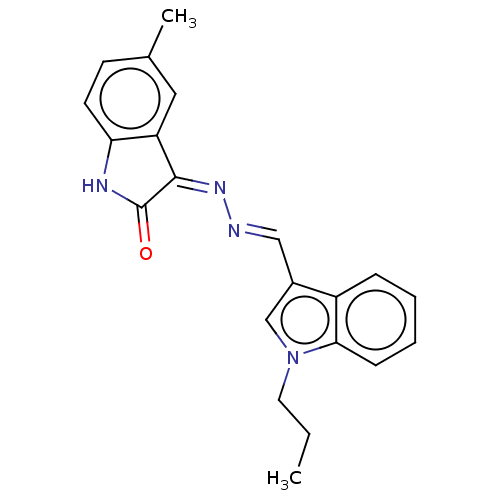
(CHEMBL5196783)Show SMILES CCCn1cc(\C=N\N=C2/C(=O)Nc3ccc(C)cc23)c2ccccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Relative binding affinity for human estrogen receptor beta by displacement of [3H]estradiol |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50410796
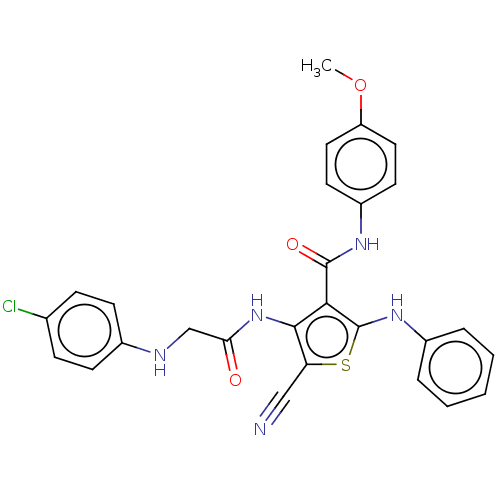
(CHEMBL5290309)Show SMILES FC(F)(F)c1cc(cc(c1)C(F)(F)F)C(=O)N1CCN(C\C=N/OCCCN2CCOCC2)C[C@H]1Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C31H35F6N5O3/c32-30(33,34)24-16-22(17-25(19-24)31(35,36)37)29(43)42-10-9-41(8-6-39-45-13-3-7-40-11-14-44-15-12-40)21-26(42)18-23-20-38-28-5-2-1-4-27(23)28/h1-2,4-6,16-17,19-20,26,38H,3,7-15,18,21H2/b39-6-/t26-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against FAAH in Wistar rat brain homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data