 Found 504 hits with Last Name = 'meng' and Initial = 'h'
Found 504 hits with Last Name = 'meng' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50234442
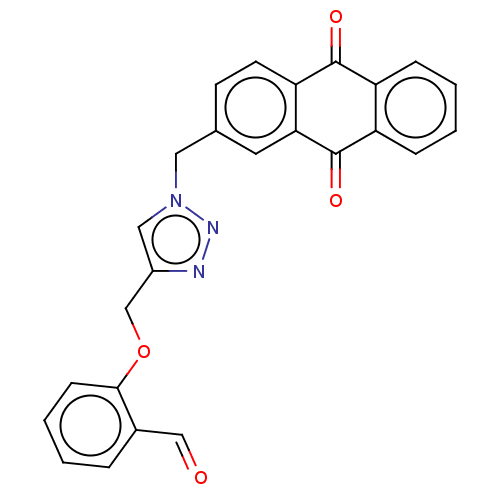
(CHEMBL4069614)Show SMILES O=Cc1ccccc1OCc1cn(Cc2ccc3C(=O)c4ccccc4C(=O)c3c2)nn1 Show InChI InChI=1S/C25H17N3O4/c29-14-17-5-1-4-8-23(17)32-15-18-13-28(27-26-18)12-16-9-10-21-22(11-16)25(31)20-7-3-2-6-19(20)24(21)30/h1-11,13-14H,12,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Medical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine xanthine oxidase assessed as enzyme-inhibitor complex using xanthine as substrate after 60 secs by Lineweaver-Burk pl... |
Bioorg Med Chem Lett 27: 729-732 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.049
BindingDB Entry DOI: 10.7270/Q2GX4DS2 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50234442

(CHEMBL4069614)Show SMILES O=Cc1ccccc1OCc1cn(Cc2ccc3C(=O)c4ccccc4C(=O)c3c2)nn1 Show InChI InChI=1S/C25H17N3O4/c29-14-17-5-1-4-8-23(17)32-15-18-13-28(27-26-18)12-16-9-10-21-22(11-16)25(31)20-7-3-2-6-19(20)24(21)30/h1-11,13-14H,12,15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Medical University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of bovine xanthine oxidase assessed as enzyme-inhibitor-substrate complex using xanthine as substrate after 60 secs by Lineweav... |
Bioorg Med Chem Lett 27: 729-732 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.049
BindingDB Entry DOI: 10.7270/Q2GX4DS2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50457170
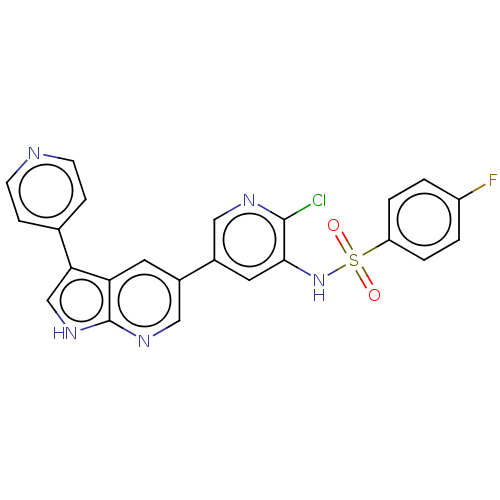
(CHEMBL4208385)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-21(30-33(31,32)18-3-1-17(25)2-4-18)10-16(11-27-22)15-9-19-20(13-29-23(19)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged PI3K p110delta/p85alpha expressed in baculovirus expression system using PIP2/PS as substrate ... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50457159

(CHEMBL4217725)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H16ClN5O2S/c24-22-21(29-32(30,31)18-4-2-1-3-5-18)11-17(12-26-22)16-10-19-20(14-28-23(19)27-13-16)15-6-8-25-9-7-15/h1-14,29H,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2/PS as substrate after 1 h... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50457173

(CHEMBL4213317)Show SMILES Fc1ccccc1S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-20(30-33(31,32)21-4-2-1-3-19(21)25)10-16(11-27-22)15-9-17-18(13-29-23(17)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged PI3K p110delta/p85alpha expressed in baculovirus expression system using PIP2/PS as substrate ... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50457159

(CHEMBL4217725)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H16ClN5O2S/c24-22-21(29-32(30,31)18-4-2-1-3-5-18)11-17(12-26-22)16-10-19-20(14-28-23(19)27-13-16)15-6-8-25-9-7-15/h1-14,29H,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged PI3K p110delta/p85alpha expressed in baculovirus expression system using PIP2/PS as substrate ... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50457173

(CHEMBL4213317)Show SMILES Fc1ccccc1S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-20(30-33(31,32)21-4-2-1-3-19(21)25)10-16(11-27-22)15-9-17-18(13-29-23(17)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2/PS as substrate after 1 h... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50457158
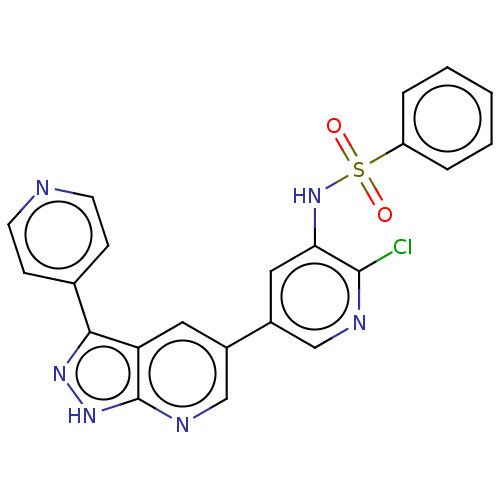
(CHEMBL4212775)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C22H15ClN6O2S/c23-21-19(29-32(30,31)17-4-2-1-3-5-17)11-16(12-25-21)15-10-18-20(14-6-8-24-9-7-14)27-28-22(18)26-13-15/h1-13,29H,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged PI3K p110delta/p85alpha expressed in baculovirus expression system using PIP2/PS as substrate ... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50457170

(CHEMBL4208385)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-21(30-33(31,32)18-3-1-17(25)2-4-18)10-16(11-27-22)15-9-19-20(13-29-23(19)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2/PS as substrate after 1 h... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50568576
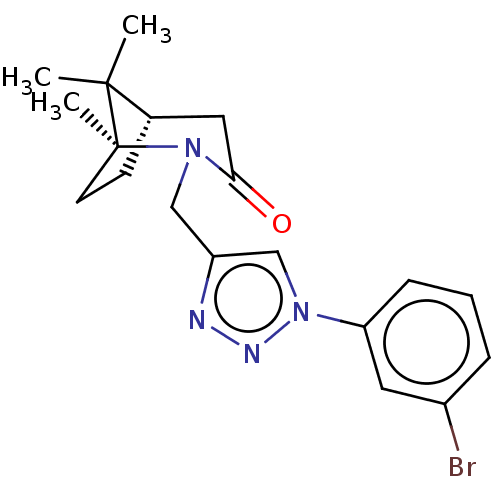
(CHEMBL4873718)Show SMILES [H][C@]12CC[C@](C)(N(Cc3cn(nn3)-c3cccc(Br)c3)C(=O)C1)C2(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N terminal his-tagged HSP90 alpha (1 to 732 residues) expressed in Escherichia coli measured after 60 mins by Floures... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112988
BindingDB Entry DOI: 10.7270/Q2RN3CMR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50457168

(CHEMBL4204586)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]cc(-c3ccccc3)c2c1 Show InChI InChI=1S/C24H17ClN4O2S/c25-23-22(29-32(30,31)19-9-5-2-6-10-19)12-18(13-26-23)17-11-20-21(15-28-24(20)27-14-17)16-7-3-1-4-8-16/h1-15,29H,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged PI3K p110delta/p85alpha expressed in baculovirus expression system using PIP2/PS as substrate ... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50457160

(CHEMBL4204119)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]ncc2c1 Show InChI InChI=1S/C17H12ClN5O2S/c18-16-15(23-26(24,25)14-4-2-1-3-5-14)7-12(8-19-16)11-6-13-10-21-22-17(13)20-9-11/h1-10,23H,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged PI3K p110delta/p85alpha expressed in baculovirus expression system using PIP2/PS as substrate ... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50457170

(CHEMBL4208385)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-21(30-33(31,32)18-3-1-17(25)2-4-18)10-16(11-27-22)15-9-19-20(13-29-23(19)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in baculovirus expression system using PIP2/PS as... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50457169
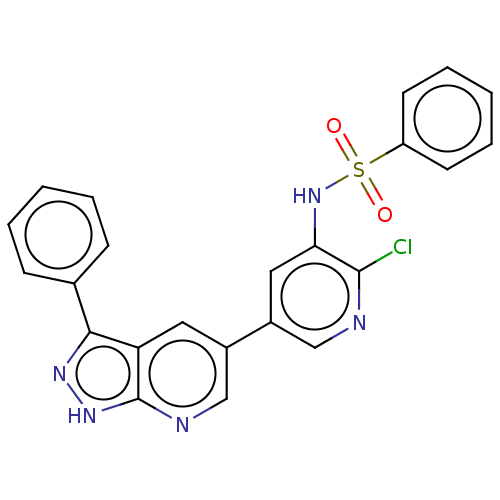
(CHEMBL4216255)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]nc(-c3ccccc3)c2c1 Show InChI InChI=1S/C23H16ClN5O2S/c24-22-20(29-32(30,31)18-9-5-2-6-10-18)12-17(13-25-22)16-11-19-21(15-7-3-1-4-8-15)27-28-23(19)26-14-16/h1-14,29H,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged PI3K p110delta/p85alpha expressed in baculovirus expression system using PIP2/PS as substrate ... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50457171
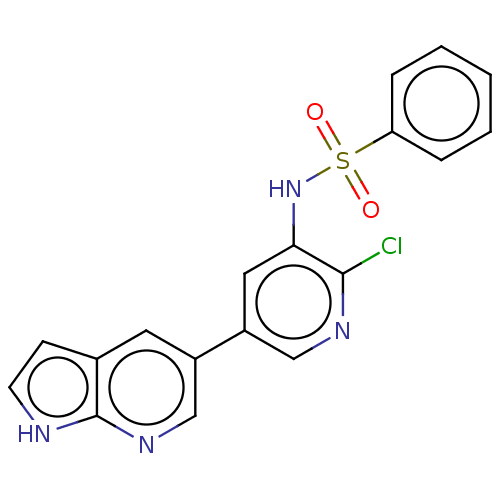
(CHEMBL4214320)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C18H13ClN4O2S/c19-17-16(23-26(24,25)15-4-2-1-3-5-15)9-14(10-21-17)13-8-12-6-7-20-18(12)22-11-13/h1-11,23H,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged PI3K p110delta/p85alpha expressed in baculovirus expression system using PIP2/PS as substrate ... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50457158

(CHEMBL4212775)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C22H15ClN6O2S/c23-21-19(29-32(30,31)17-4-2-1-3-5-17)11-16(12-25-21)15-10-18-20(14-6-8-24-9-7-14)27-28-22(18)26-13-15/h1-13,29H,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in baculovirus expression system using PIP2/PS as... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50457159

(CHEMBL4217725)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H16ClN5O2S/c24-22-21(29-32(30,31)18-4-2-1-3-5-18)11-17(12-26-22)16-10-19-20(14-28-23(19)27-13-16)15-6-8-25-9-7-15/h1-14,29H,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in baculovirus expression system using PIP2/PS as... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50457173

(CHEMBL4213317)Show SMILES Fc1ccccc1S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-20(30-33(31,32)21-4-2-1-3-19(21)25)10-16(11-27-22)15-9-17-18(13-29-23(17)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in baculovirus expression system using PIP2/PS as... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50152769
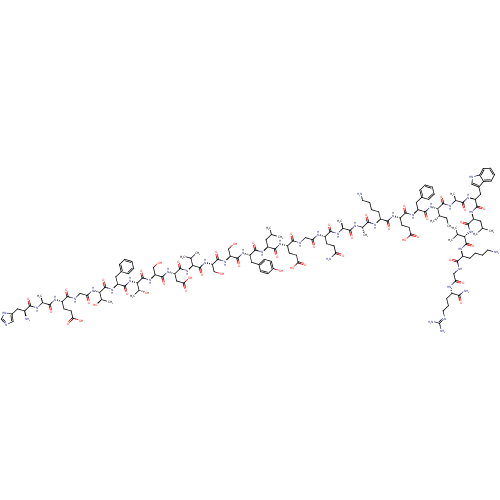
(CHEMBL410972 | GLP-1(7-36)-NH2 | GLP-17-(7-36) der...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:156.159,142.150,134.137,164.169,119.121,167.172,105.111,95.99,77.87,60.66,47.53,37.38,19.25,4.4,181.184,203.208,wD:151.154,123.133,113.117,101.103,89.93,69.74,42.43,28.34,8.16,2.2,176.180,195.200,210.215,223.228,(82.22,1.34,;82.22,-.2,;83.55,-.98,;84.89,-.21,;83.55,-2.52,;82.22,-3.29,;80.88,-2.53,;80.88,-.98,;79.55,-3.29,;79.55,-4.83,;80.88,-5.61,;82.22,-4.83,;83.55,-5.6,;83.56,-7.14,;82.23,-7.91,;80.89,-7.15,;78.21,-2.53,;76.87,-3.3,;76.87,-4.84,;75.55,-2.53,;75.55,-.99,;76.87,-.21,;76.87,1.33,;75.55,2.1,;78.21,2.09,;74.21,-3.3,;72.88,-2.53,;72.88,-.99,;71.54,-3.3,;71.54,-4.84,;72.88,-5.62,;72.88,-7.16,;74.21,-7.92,;74.21,-9.46,;70.2,-2.54,;68.87,-3.31,;68.87,-4.84,;67.53,-2.54,;67.53,-1,;66.21,-3.31,;64.87,-2.54,;64.87,-1,;63.53,-3.31,;63.53,-4.85,;62.2,-2.55,;60.86,-3.32,;60.86,-4.85,;59.53,-2.55,;59.53,-1.01,;60.86,-.23,;60.86,1.31,;59.52,2.08,;62.19,2.08,;58.19,-3.32,;56.86,-2.55,;56.86,-1.01,;55.53,-3.32,;54.19,-2.56,;52.86,-3.32,;52.86,-4.86,;51.52,-2.56,;51.52,-1.01,;52.86,-.24,;52.86,1.3,;51.51,2.07,;54.18,2.07,;50.19,-3.33,;48.85,-2.56,;48.85,-1.02,;47.51,-3.33,;47.51,-4.87,;48.85,-5.64,;48.85,-7.18,;50.19,-4.87,;46.18,-2.56,;44.85,-3.33,;44.85,-4.87,;43.52,-2.57,;43.52,-1.02,;44.85,-.25,;46.18,-1.02,;47.51,-.24,;47.5,1.3,;48.84,2.06,;46.17,2.06,;44.84,1.29,;42.18,-3.34,;40.84,-2.57,;40.84,-1.03,;39.51,-3.34,;39.51,-4.88,;40.84,-5.65,;38.17,-2.57,;36.84,-3.34,;36.84,-4.88,;35.5,-2.58,;35.5,-1.03,;36.84,-.26,;34.17,-3.35,;32.84,-2.58,;32.84,-1.04,;31.49,-3.35,;30.17,-2.58,;28.82,-3.35,;28.82,-4.89,;27.5,-2.59,;27.5,-1.04,;28.82,-.27,;30.16,-1.04,;28.82,1.28,;26.15,-3.36,;24.83,-2.6,;24.83,-1.05,;23.49,-3.37,;23.49,-4.9,;24.83,-5.68,;22.16,-2.6,;20.82,-3.37,;20.82,-4.91,;19.49,-2.6,;18.14,-3.37,;16.82,-2.61,;16.82,-1.06,;15.47,-3.37,;15.47,-4.91,;16.82,-5.69,;18.15,-4.91,;19.49,-5.68,;19.49,-7.22,;18.15,-7.99,;16.82,-7.23,;14.15,-2.61,;12.81,-3.38,;12.81,-4.92,;11.48,-2.61,;10.14,-3.38,;8.81,-2.62,;8.81,-1.07,;7.47,-3.38,;6.14,-2.62,;4.79,-3.39,;4.79,-4.92,;3.47,-2.62,;3.47,-1.08,;4.79,-.3,;4.79,1.24,;3.47,2.01,;6.13,2.01,;2.13,-3.39,;.8,-2.62,;.8,-1.08,;-.54,-3.39,;-.54,-4.93,;-1.87,-2.63,;-3.21,-3.39,;-3.21,-4.93,;-4.55,-2.63,;-5.87,-3.39,;-4.55,-1.09,;-3.21,-.31,;-1.8,-.94,;-.77,.21,;-1.55,1.55,;-3.05,1.22,;11.48,-1.07,;10.14,-.29,;12.81,-.3,;19.49,-1.05,;18.14,-.28,;20.82,-.28,;31.49,-4.89,;32.84,-5.66,;30.17,-5.66,;84.89,-3.29,;84.89,-4.83,;86.23,-2.52,;87.55,-3.28,;87.55,-4.82,;88.89,-2.52,;88.89,-.96,;90.22,-3.28,;91.56,-2.51,;91.56,-.96,;92.89,-.19,;94.3,-.81,;95.34,.33,;94.56,1.66,;95.03,3.13,;94,4.28,;92.5,3.96,;92.02,2.49,;93.05,1.35,;92.89,-3.28,;92.89,-4.81,;94.23,-2.51,;95.57,-3.27,;95.57,-4.81,;96.9,-5.58,;96.9,-7.12,;98.24,-4.81,;96.9,-2.5,;96.9,-.96,;98.23,-3.26,;99.57,-2.5,;99.57,-.95,;100.9,-.18,;98.23,-.18,;100.9,-3.26,;100.9,-4.8,;102.24,-2.49,;103.57,-3.26,;103.57,-4.8,;104.91,-5.57,;104.91,-7.11,;106.25,-7.88,;106.25,-9.42,;104.91,-2.49,;104.91,-.95,;106.24,-3.26,;107.58,-2.49,;108.91,-3.25,;108.91,-4.79,;110.25,-2.48,;111.58,-3.25,;111.58,-4.79,;112.92,-5.56,;112.92,-7.1,;114.26,-7.87,;114.26,-9.41,;112.92,-10.18,;115.6,-10.18,;112.92,-2.48,;114.25,-3.25,;112.92,-.94,)| Show InChI InChI=1S/C149H226N40O45/c1-17-76(10)119(146(232)167-80(14)126(212)175-104(60-86-63-159-91-36-25-24-35-89(86)91)136(222)177-100(56-73(4)5)137(223)186-117(74(6)7)144(230)174-93(37-26-28-52-150)128(214)160-65-110(197)168-92(122(154)208)39-30-54-158-149(155)156)188-138(224)102(57-83-31-20-18-21-32-83)178-133(219)98(47-51-115(204)205)173-132(218)94(38-27-29-53-151)170-124(210)78(12)164-123(209)77(11)166-131(217)97(44-48-109(153)196)169-111(198)66-161-130(216)96(46-50-114(202)203)172-134(220)99(55-72(2)3)176-135(221)101(59-85-40-42-88(195)43-41-85)179-141(227)106(68-190)182-143(229)108(70-192)183-145(231)118(75(8)9)187-140(226)105(62-116(206)207)180-142(228)107(69-191)184-148(234)121(82(16)194)189-139(225)103(58-84-33-22-19-23-34-84)181-147(233)120(81(15)193)185-112(199)67-162-129(215)95(45-49-113(200)201)171-125(211)79(13)165-127(213)90(152)61-87-64-157-71-163-87/h18-25,31-36,40-43,63-64,71-82,90,92-108,117-121,159,190-195H,17,26-30,37-39,44-62,65-70,150-152H2,1-16H3,(H2,153,196)(H2,154,208)(H,157,163)(H,160,214)(H,161,216)(H,162,215)(H,164,209)(H,165,213)(H,166,217)(H,167,232)(H,168,197)(H,169,198)(H,170,210)(H,171,211)(H,172,220)(H,173,218)(H,174,230)(H,175,212)(H,176,221)(H,177,222)(H,178,219)(H,179,227)(H,180,228)(H,181,233)(H,182,229)(H,183,231)(H,184,234)(H,185,199)(H,186,223)(H,187,226)(H,188,224)(H,189,225)(H,200,201)(H,202,203)(H,204,205)(H,206,207)(H4,155,156,158)/t76-,77-,78-,79-,80-,81+,82+,90-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,117-,118-,119-,120-,121-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Displacement of [125I]exendin(9-39) from human GLP1R expressed in CHO cells |
J Med Chem 51: 7303-7 (2009)
Article DOI: 10.1021/jm8008579
BindingDB Entry DOI: 10.7270/Q2MS3SN9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50457159

(CHEMBL4217725)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H16ClN5O2S/c24-22-21(29-32(30,31)18-4-2-1-3-5-18)11-17(12-26-22)16-10-19-20(14-28-23(19)27-13-16)15-6-8-25-9-7-15/h1-14,29H,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3K p110beta using PIP2/PS as substrate after 1 hr by ADP-Glo luminescence assay |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50457169

(CHEMBL4216255)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]nc(-c3ccccc3)c2c1 Show InChI InChI=1S/C23H16ClN5O2S/c24-22-20(29-32(30,31)18-9-5-2-6-10-18)12-17(13-25-22)16-11-19-21(15-7-3-1-4-8-15)27-28-23(19)26-14-16/h1-14,29H,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in baculovirus expression system using PIP2/PS as... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50457168

(CHEMBL4204586)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]cc(-c3ccccc3)c2c1 Show InChI InChI=1S/C24H17ClN4O2S/c25-23-22(29-32(30,31)19-9-5-2-6-10-19)12-18(13-26-23)17-11-20-21(15-28-24(20)27-14-17)16-7-3-1-4-8-16/h1-15,29H,(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in baculovirus expression system using PIP2/PS as... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50457170

(CHEMBL4208385)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-21(30-33(31,32)18-3-1-17(25)2-4-18)10-16(11-27-22)15-9-19-20(13-29-23(19)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3K p110beta using PIP2/PS as substrate after 1 hr by ADP-Glo luminescence assay |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
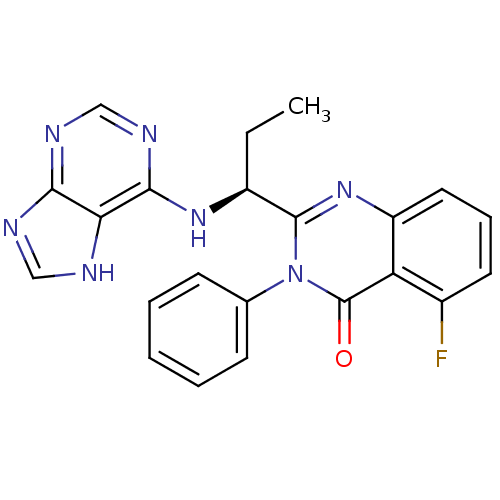
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of human full length His-tagged PI3Kdelta expressed in baculovirus expression system |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50568573
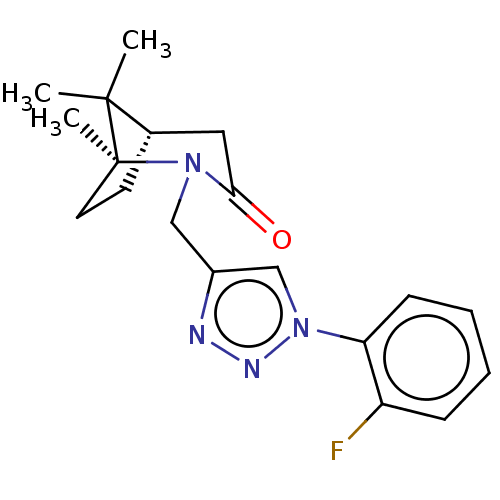
(CHEMBL4854592)Show SMILES [H][C@]12CC[C@](C)(N(Cc3cn(nn3)-c3ccccc3F)C(=O)C1)C2(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N terminal his-tagged HSP90 alpha (1 to 732 residues) expressed in Escherichia coli measured after 60 mins by Floures... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112988
BindingDB Entry DOI: 10.7270/Q2RN3CMR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50457173

(CHEMBL4213317)Show SMILES Fc1ccccc1S(=O)(=O)Nc1cc(cnc1Cl)-c1cnc2[nH]cc(-c3ccncc3)c2c1 Show InChI InChI=1S/C23H15ClFN5O2S/c24-22-20(30-33(31,32)21-4-2-1-3-19(21)25)10-16(11-27-22)15-9-17-18(13-29-23(17)28-12-15)14-5-7-26-8-6-14/h1-13,30H,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3K p110beta using PIP2/PS as substrate after 1 hr by ADP-Glo luminescence assay |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50259731
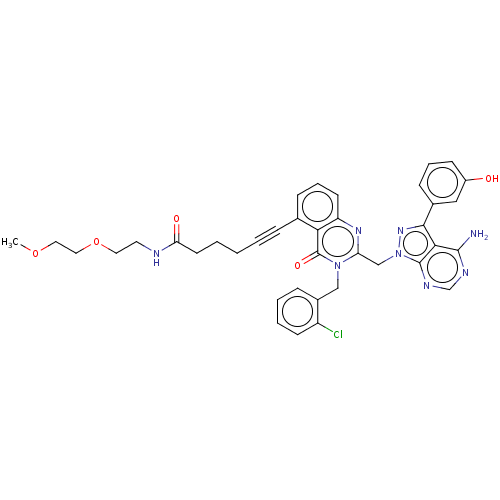
(CHEMBL4075540)Show SMILES COCCOCCNC(=O)CCCC#Cc1cccc2nc(Cn3nc(-c4cccc(O)c4)c4c(N)ncnc34)n(Cc3ccccc3Cl)c(=O)c12 Show InChI InChI=1S/C38H37ClN8O5/c1-51-19-20-52-18-17-41-32(49)16-4-2-3-9-25-11-8-15-30-33(25)38(50)46(22-27-10-5-6-14-29(27)39)31(44-30)23-47-37-34(36(40)42-24-43-37)35(45-47)26-12-7-13-28(48)21-26/h5-8,10-15,21,24,48H,2,4,16-20,22-23H2,1H3,(H,41,49)(H2,40,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotinylated PIP2 as substrate after 2 hrs by TR-FRET assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50568574
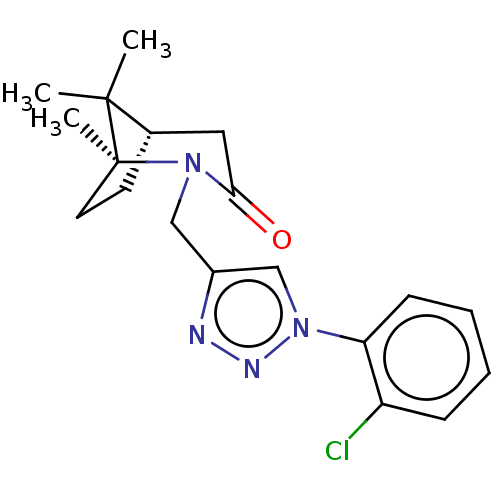
(CHEMBL4849823)Show SMILES [H][C@]12CC[C@](C)(N(Cc3cn(nn3)-c3ccccc3Cl)C(=O)C1)C2(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N terminal his-tagged HSP90 alpha (1 to 732 residues) expressed in Escherichia coli measured after 60 mins by Floures... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112988
BindingDB Entry DOI: 10.7270/Q2RN3CMR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50259733
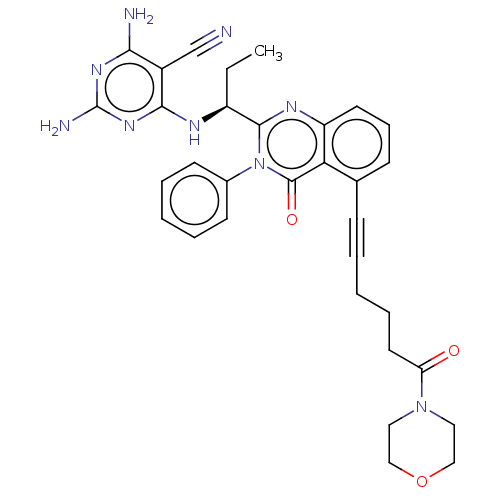
(CHEMBL4073929)Show SMILES CC[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(C#CCCCC(=O)N3CCOCC3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C32H33N9O3/c1-2-24(36-29-23(20-33)28(34)38-32(35)39-29)30-37-25-14-9-11-21(27(25)31(43)41(30)22-12-6-4-7-13-22)10-5-3-8-15-26(42)40-16-18-44-19-17-40/h4,6-7,9,11-14,24H,2-3,8,15-19H2,1H3,(H5,34,35,36,38,39)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 30 mins by HTRF assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50457158

(CHEMBL4212775)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C22H15ClN6O2S/c23-21-19(29-32(30,31)17-4-2-1-3-5-17)11-16(12-25-21)15-10-18-20(14-6-8-24-9-7-14)27-28-22(18)26-13-15/h1-13,29H,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3K p110beta using PIP2/PS as substrate after 1 hr by ADP-Glo luminescence assay |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068

(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 30 mins by HTRF assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50275523
(CHEMBL500187)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CC(C(F)(F)F)C(F)(F)F)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C152H227F6N41O44/c1-17-76(10)120(147(241)177-80(14)127(221)184-103(58-86-62-168-91-36-25-24-35-89(86)91)137(231)187-99(54-73(4)5)138(232)196-118(74(6)7)145(239)183-93(37-26-28-50-159)129(223)169-64-111(207)178-92(123(163)217)39-30-52-167-150(164)165)198-139(233)101(55-83-31-20-18-21-32-83)188-135(229)97(46-49-116(213)214)182-134(228)94(38-27-29-51-160)181-125(219)78(12)174-124(218)77(11)176-133(227)96(44-47-110(162)206)180-113(209)65-170-130(224)95(45-48-115(211)212)179-112(208)66-171-131(225)98(53-72(2)3)186-136(230)100(57-85-40-42-88(205)43-41-85)189-142(236)106(68-200)192-144(238)108(70-202)193-146(240)119(75(8)9)197-141(235)105(61-117(215)216)190-143(237)107(69-201)194-149(243)122(82(16)204)199-140(234)102(56-84-33-22-19-23-34-84)191-148(242)121(81(15)203)195-114(210)67-172-132(226)104(60-109(151(153,154)155)152(156,157)158)185-126(220)79(13)175-128(222)90(161)59-87-63-166-71-173-87/h18-25,31-36,40-43,62-63,71-82,90,92-109,118-122,168,200-205H,17,26-30,37-39,44-61,64-70,159-161H2,1-16H3,(H2,162,206)(H2,163,217)(H,166,173)(H,169,223)(H,170,224)(H,171,225)(H,172,226)(H,174,218)(H,175,222)(H,176,227)(H,177,241)(H,178,207)(H,179,208)(H,180,209)(H,181,219)(H,182,228)(H,183,239)(H,184,221)(H,185,220)(H,186,230)(H,187,231)(H,188,229)(H,189,236)(H,190,237)(H,191,242)(H,192,238)(H,193,240)(H,194,243)(H,195,210)(H,196,232)(H,197,235)(H,198,233)(H,199,234)(H,211,212)(H,213,214)(H,215,216)(H4,164,165,167)/t76-,77-,78-,79-,80-,81+,82+,90-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,107-,108-,118-,119-,120-,121-,122-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Displacement of [125I]exendin(9-39) from human GLP1R expressed in CHO cells |
J Med Chem 51: 7303-7 (2009)
Article DOI: 10.1021/jm8008579
BindingDB Entry DOI: 10.7270/Q2MS3SN9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM330295
(Methyl (5-(6-((4-(methylsulfonyl)piperazin-1-yl)me...)Show SMILES COC(=O)Nc1cc(c(cn1)-c1nc(N2CCOCC2)c2cc(CN3CCN(CC3)S(C)(=O)=O)cn2n1)C(F)(F)F Show InChI InChI=1S/C24H29F3N8O5S/c1-39-23(36)29-20-12-18(24(25,26)27)17(13-28-20)21-30-22(33-7-9-40-10-8-33)19-11-16(15-35(19)31-21)14-32-3-5-34(6-4-32)41(2,37)38/h11-13,15H,3-10,14H2,1-2H3,(H,28,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PI3Kalpha using PIP2 as substrate in presence of ATP measured after 30 mins by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112913
BindingDB Entry DOI: 10.7270/Q2TF0228 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50457169

(CHEMBL4216255)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]nc(-c3ccccc3)c2c1 Show InChI InChI=1S/C23H16ClN5O2S/c24-22-20(29-32(30,31)18-9-5-2-6-10-18)12-17(13-25-22)16-11-19-21(15-7-3-1-4-8-15)27-28-23(19)26-14-16/h1-14,29H,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PI3K p110beta using PIP2/PS as substrate after 1 hr by ADP-Glo luminescence assay |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50259729
(CHEMBL4060396)Show SMILES CC[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(C#CCCCC(=O)N3CCCC3)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C32H33N9O2/c1-2-24(36-29-23(20-33)28(34)38-32(35)39-29)30-37-25-16-11-13-21(12-5-3-8-17-26(42)40-18-9-10-19-40)27(25)31(43)41(30)22-14-6-4-7-15-22/h4,6-7,11,13-16,24H,2-3,8-10,17-19H2,1H3,(H5,34,35,36,38,39)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 30 mins by HTRF assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50457158

(CHEMBL4212775)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C22H15ClN6O2S/c23-21-19(29-32(30,31)17-4-2-1-3-5-17)11-16(12-25-21)15-10-18-20(14-6-8-24-9-7-14)27-28-22(18)26-13-15/h1-13,29H,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2/PS as substrate after 1 h... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50259740
(CHEMBL4098370)Show SMILES Cc1nc(N)nc(N2CCC[C@H]2c2nc3cccc(C#CCCCC(=O)N4CCCC4)c3c(=O)n2-c2ccccc2)c1C#N |r| Show InChI InChI=1S/C34H34N8O2/c1-23-26(22-35)31(39-34(36)37-23)41-21-11-17-28(41)32-38-27-16-10-13-24(12-4-2-7-18-29(43)40-19-8-9-20-40)30(27)33(44)42(32)25-14-5-3-6-15-25/h3,5-6,10,13-16,28H,2,7-9,11,17-21H2,1H3,(H2,36,37,39)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 30 mins by HTRF assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50568575
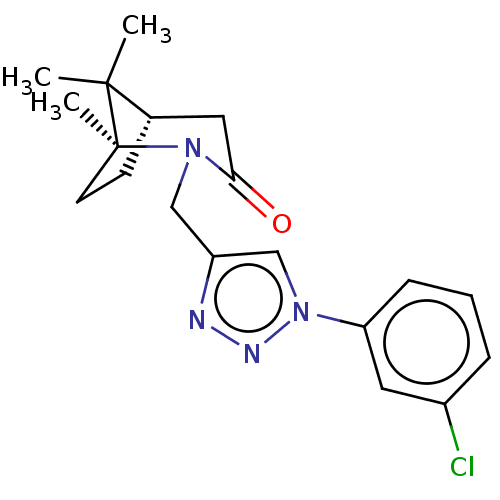
(CHEMBL4857130)Show SMILES [H][C@]12CC[C@](C)(N(Cc3cn(nn3)-c3cccc(Cl)c3)C(=O)C1)C2(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N terminal his-tagged HSP90 alpha (1 to 732 residues) expressed in Escherichia coli measured after 60 mins by Floures... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112988
BindingDB Entry DOI: 10.7270/Q2RN3CMR |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha
(Homo sapiens (Human)) | BDBM50568571
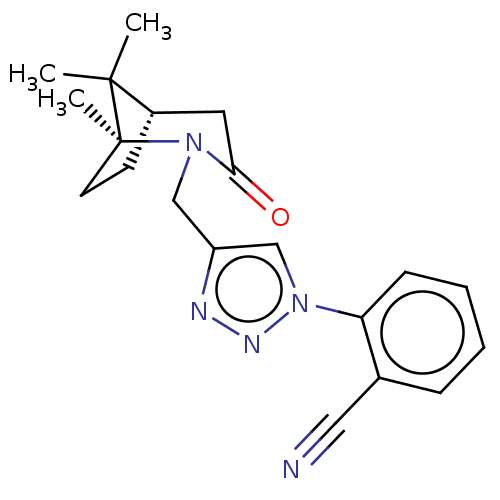
(CHEMBL4853099)Show SMILES [H][C@]12CC[C@](C)(N(Cc3cn(nn3)-c3ccccc3C#N)C(=O)C1)C2(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N terminal his-tagged HSP90 alpha (1 to 732 residues) expressed in Escherichia coli measured after 60 mins by Floures... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112988
BindingDB Entry DOI: 10.7270/Q2RN3CMR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Central South University
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110delta by HTRF assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127194
BindingDB Entry DOI: 10.7270/Q20Z76VM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085290
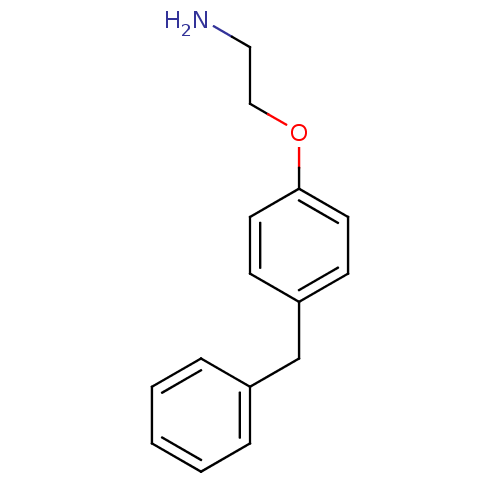
(2-(4-Benzyl-phenoxy)-ethylamine | 2-(4-benzylpheno...)Show InChI InChI=1S/C15H17NO/c16-10-11-17-15-8-6-14(7-9-15)12-13-4-2-1-3-5-13/h1-9H,10-12,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of epoxide hydrolase activity of human recombinant leukotriene A4 hydrolase expressed in Escherichia coli assessed as LTB4 production by R... |
Bioorg Med Chem Lett 18: 6549-52 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.044
BindingDB Entry DOI: 10.7270/Q2KS6RDK |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human IDO1 expressed in HEK293T cells assessed as reduction in N-formylkynurenine production incubated for 12 hrs by microplate reader ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112059
BindingDB Entry DOI: 10.7270/Q2PN997N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50259741
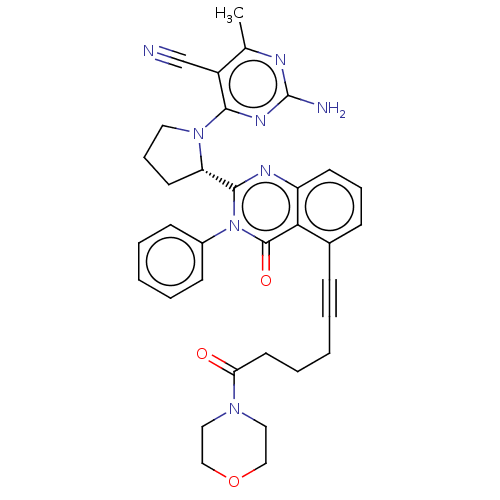
(CHEMBL4093653)Show SMILES Cc1nc(N)nc(N2CCC[C@H]2c2nc3cccc(C#CCCCC(=O)N4CCOCC4)c3c(=O)n2-c2ccccc2)c1C#N |r| Show InChI InChI=1S/C34H34N8O3/c1-23-26(22-35)31(39-34(36)37-23)41-17-9-15-28(41)32-38-27-14-8-11-24(30(27)33(44)42(32)25-12-5-3-6-13-25)10-4-2-7-16-29(43)40-18-20-45-21-19-40/h3,5-6,8,11-14,28H,2,7,9,15-21H2,1H3,(H2,36,37,39)/t28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 30 mins by HTRF assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50259734
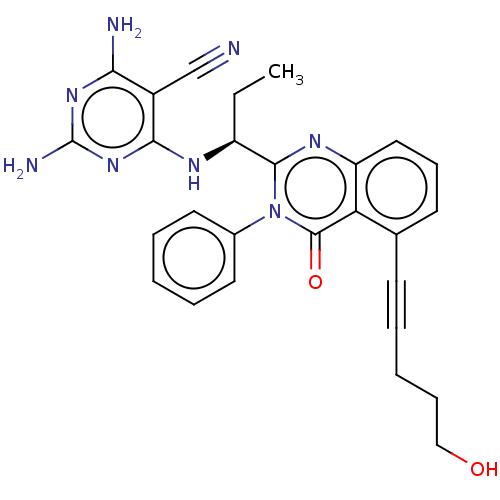
(CHEMBL4087312)Show SMILES CC[C@H](Nc1nc(N)nc(N)c1C#N)c1nc2cccc(C#CCCCO)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C27H26N8O2/c1-2-20(31-24-19(16-28)23(29)33-27(30)34-24)25-32-21-14-9-11-17(10-5-4-8-15-36)22(21)26(37)35(25)18-12-6-3-7-13-18/h3,6-7,9,11-14,20,36H,2,4,8,15H2,1H3,(H5,29,30,31,33,34)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 30 mins by HTRF assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50259739
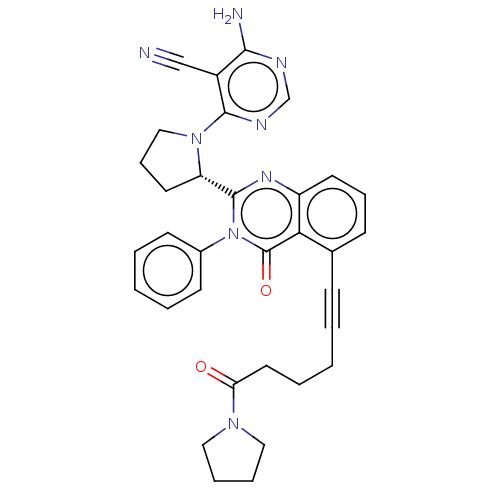
(CHEMBL4092557)Show SMILES Nc1ncnc(N2CCC[C@H]2c2nc3cccc(C#CCCCC(=O)N4CCCC4)c3c(=O)n2-c2ccccc2)c1C#N |r| Show InChI InChI=1S/C33H32N8O2/c34-21-25-30(35)36-22-37-31(25)40-20-10-16-27(40)32-38-26-15-9-12-23(11-3-1-6-17-28(42)39-18-7-8-19-39)29(26)33(43)41(32)24-13-4-2-5-14-24/h2,4-5,9,12-15,22,27H,1,6-8,10,16-20H2,(H2,35,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 30 mins by HTRF assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | |
Glucagon-like peptide 1 receptor
(Homo sapiens (Human)) | BDBM50275527
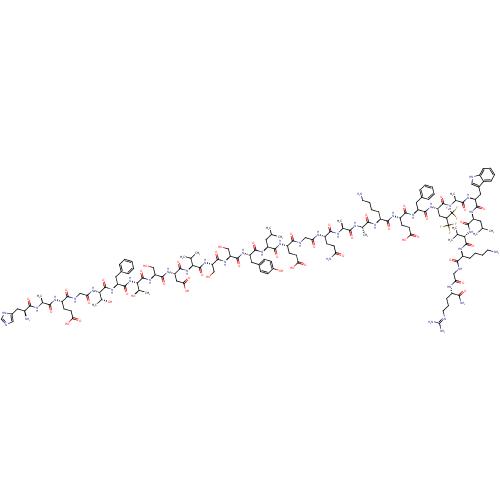
(CHEMBL524875)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1cnc[nH]1)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C(F)(F)F)C(F)(F)F)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CCCN=C(N)N)C(N)=O |r,wU:87.89,73.80,65.67,95.99,50.51,98.102,36.41,26.29,8.17,107.109,120.122,134.137,148.150,187.190,209.214,wD:82.84,54.63,44.47,32.33,20.23,4.4,129.132,139.141,157.159,168.171,182.186,201.206,216.221,229.234,(45.55,-6.81,;45.55,-5.27,;46.89,-4.5,;44.21,-4.5,;44.21,-2.96,;42.88,-2.19,;41.54,-2.96,;41.54,-4.5,;40.21,-2.19,;40.21,-.65,;41.54,.12,;42.86,-.64,;44.19,.13,;44.19,1.67,;45.52,2.44,;42.85,2.44,;41.53,1.66,;38.87,-2.97,;37.54,-2.2,;37.54,-.65,;36.21,-2.97,;36.21,-4.51,;37.54,-5.28,;34.87,-2.2,;33.53,-2.97,;33.53,-4.51,;32.21,-2.2,;32.21,-.66,;33.53,.11,;30.87,-2.97,;29.54,-2.21,;29.54,-.66,;28.2,-2.98,;26.87,-2.21,;25.53,-2.98,;25.53,-4.52,;24.2,-2.21,;24.2,-.67,;25.53,.1,;26.87,-.67,;25.53,1.64,;22.87,-2.98,;21.53,-2.22,;21.53,-.67,;20.2,-2.99,;20.2,-4.52,;21.53,-5.3,;18.87,-2.22,;17.53,-2.99,;17.53,-4.53,;16.2,-2.22,;14.86,-2.99,;13.53,-2.22,;13.53,-.68,;12.19,-3,;12.19,-4.53,;13.53,-5.3,;14.85,-4.53,;16.19,-5.3,;16.19,-6.85,;14.85,-7.61,;13.53,-6.84,;10.87,-2.23,;9.53,-3,;9.53,-4.54,;8.19,-2.23,;6.86,-3,;5.53,-2.24,;5.53,-.69,;4.2,-3.01,;2.86,-2.24,;1.52,-3.02,;1.52,-4.55,;.19,-2.25,;.19,-.7,;1.52,.06,;1.52,1.61,;.19,2.38,;2.86,2.38,;-1.14,-3.02,;-2.47,-2.25,;-2.47,-.71,;-3.81,-3.02,;-3.81,-4.56,;-5.15,-2.25,;-6.48,-3.02,;-6.48,-4.56,;-7.81,-2.25,;-9.14,-3.02,;-7.81,-.71,;-6.48,.06,;-5.07,-.56,;-4.04,.58,;-4.81,1.92,;-6.32,1.59,;8.19,-.69,;6.86,.08,;9.53,.08,;16.2,-.68,;14.86,.09,;17.53,.09,;28.2,-4.51,;29.54,-5.29,;26.87,-5.28,;45.55,-2.19,;45.55,-.64,;46.87,-2.96,;48.21,-2.18,;48.21,-.64,;49.55,.13,;49.55,1.67,;48.21,2.44,;50.87,2.44,;49.55,-2.95,;49.55,-4.49,;50.88,-2.18,;52.21,-2.95,;53.55,-2.18,;53.55,-.64,;54.88,-2.95,;56.22,-2.18,;56.22,-.63,;57.55,.14,;57.55,1.68,;56.21,2.45,;58.88,2.45,;57.55,-2.94,;57.55,-4.48,;58.89,-2.17,;60.21,-2.94,;60.21,-4.48,;61.55,-2.17,;61.55,-.63,;62.89,-2.94,;64.22,-2.17,;64.22,-.62,;65.55,-2.93,;65.55,-4.47,;66.88,-2.16,;68.22,-2.93,;68.22,-4.47,;69.56,-5.24,;69.56,-6.78,;70.89,-7.55,;70.89,-9.09,;69.56,-2.16,;69.56,-.62,;70.89,-2.93,;72.23,-2.16,;72.23,-.61,;73.55,.15,;73.55,1.71,;72.22,2.48,;74.89,2.47,;73.55,-2.93,;73.55,-4.46,;74.89,-2.15,;76.22,-2.91,;76.22,-4.45,;77.56,-5.22,;78.9,-4.45,;80.23,-5.22,;80.23,-6.76,;78.9,-7.53,;77.56,-6.76,;77.56,-2.14,;78.89,-2.91,;77.56,-.6,;78.89,.17,;80.62,-.83,;81.95,-.06,;83.29,-.83,;84.63,-1.6,;82.52,-2.16,;84.06,.51,;81.95,1.48,;81.95,3.02,;83.49,1.48,;80.41,1.48,;78.89,1.71,;80.22,2.48,;77.55,2.48,;77.55,4.02,;78.89,4.79,;76.21,4.79,;74.88,4.02,;76.21,6.33,;74.88,7.1,;73.54,6.33,;72.21,7.1,;72.05,8.63,;70.54,8.95,;69.77,7.62,;68.26,7.29,;67.79,5.82,;68.82,4.67,;70.33,5,;70.8,6.46,;74.88,8.65,;76.21,9.42,;73.54,9.42,;73.54,10.96,;74.88,11.73,;74.88,13.27,;76.2,14.04,;73.54,14.04,;72.2,11.73,;70.87,10.96,;72.2,13.27,;70.87,14.04,;69.53,13.27,;68.2,14.03,;69.53,11.71,;70.87,15.57,;72.2,16.34,;69.53,16.35,;69.53,17.88,;70.86,18.65,;70.86,20.18,;72.19,20.95,;72.19,22.5,;73.53,23.26,;68.2,18.65,;66.86,17.88,;68.2,20.17,;66.86,20.95,;66.86,22.48,;68.19,23.25,;65.52,23.25,;65.52,24.79,;66.85,25.56,;66.85,27.1,;68.18,27.87,;68.18,29.42,;69.51,30.18,;70.85,29.42,;69.51,31.73,;64.19,25.56,;64.19,27.1,;62.85,24.79,)| Show InChI InChI=1S/C149H220F6N40O45/c1-70(2)52-96(182-134(228)99(56-82-37-39-85(201)40-38-82)185-140(234)104(66-196)189-142(236)106(68-198)190-144(238)117(73(7)8)194-139(233)103(60-115(212)213)187-141(235)105(67-197)191-146(240)119(79(14)200)195-138(232)100(55-81-30-19-16-20-31-81)188-145(239)118(78(13)199)192-111(205)65-168-127(221)92(42-46-112(206)207)177-123(217)76(11)171-125(219)87(158)58-84-62-163-69-169-84)133(227)178-93(43-47-113(208)209)128(222)167-64-110(204)175-94(41-45-108(159)202)129(223)172-74(9)121(215)170-75(10)122(216)176-91(35-24-26-50-157)130(224)179-95(44-48-114(210)211)131(225)184-98(54-80-28-17-15-18-29-80)135(229)186-102(59-107(148(150,151)152)149(153,154)155)132(226)173-77(12)124(218)181-101(57-83-61-165-88-33-22-21-32-86(83)88)136(230)183-97(53-71(3)4)137(231)193-116(72(5)6)143(237)180-90(34-23-25-49-156)126(220)166-63-109(203)174-89(120(160)214)36-27-51-164-147(161)162/h15-22,28-33,37-40,61-62,69-79,87,89-107,116-119,165,196-201H,23-27,34-36,41-60,63-68,156-158H2,1-14H3,(H2,159,202)(H2,160,214)(H,163,169)(H,166,220)(H,167,222)(H,168,221)(H,170,215)(H,171,219)(H,172,223)(H,173,226)(H,174,203)(H,175,204)(H,176,216)(H,177,217)(H,178,227)(H,179,224)(H,180,237)(H,181,218)(H,182,228)(H,183,230)(H,184,225)(H,185,234)(H,186,229)(H,187,235)(H,188,239)(H,189,236)(H,190,238)(H,191,240)(H,192,205)(H,193,231)(H,194,233)(H,195,232)(H,206,207)(H,208,209)(H,210,211)(H,212,213)(H4,161,162,164)/t74-,75-,76-,77-,78+,79+,87-,89-,90-,91-,92-,93-,94-,95-,96-,97-,98-,99-,100-,101-,102-,103-,104-,105-,106-,116-,117-,118-,119-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University
Curated by ChEMBL
| Assay Description
Displacement of [125I]exendin(9-39) from human GLP1R expressed in CHO cells |
J Med Chem 51: 7303-7 (2009)
Article DOI: 10.1021/jm8008579
BindingDB Entry DOI: 10.7270/Q2MS3SN9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50259730
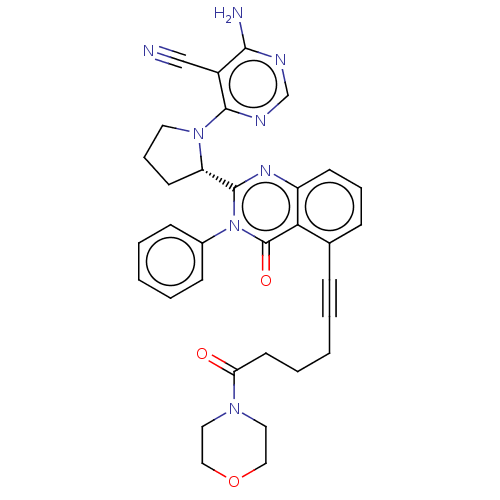
(CHEMBL4080347)Show SMILES Nc1ncnc(N2CCC[C@H]2c2nc3cccc(C#CCCCC(=O)N4CCOCC4)c3c(=O)n2-c2ccccc2)c1C#N |r| Show InChI InChI=1S/C33H32N8O3/c34-21-25-30(35)36-22-37-31(25)40-16-8-14-27(40)32-38-26-13-7-10-23(29(26)33(43)41(32)24-11-4-2-5-12-24)9-3-1-6-15-28(42)39-17-19-44-20-18-39/h2,4-5,7,10-13,22,27H,1,6,8,14-20H2,(H2,35,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica (SIMM)
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate measured after 30 mins by HTRF assay |
Eur J Med Chem 125: 1156-1171 (2017)
Article DOI: 10.1016/j.ejmech.2016.11.014
BindingDB Entry DOI: 10.7270/Q2FF3VTC |
More data for this
Ligand-Target Pair | |
Deoxyhypusine synthase
(Homo sapiens) | BDBM50583904
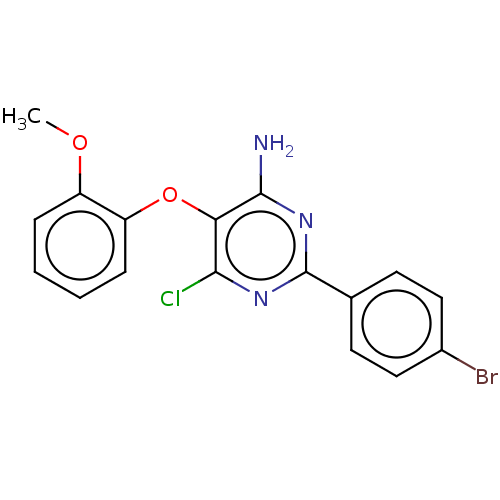
(CHEMBL5091313) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DHPS (unknown origin ) incubated for 30 mins by NAD/NADH-Glow assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00582
BindingDB Entry DOI: 10.7270/Q2BV7MH5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50457168

(CHEMBL4204586)Show SMILES Clc1ncc(cc1NS(=O)(=O)c1ccccc1)-c1cnc2[nH]cc(-c3ccccc3)c2c1 Show InChI InChI=1S/C24H17ClN4O2S/c25-23-22(29-32(30,31)19-9-5-2-6-10-19)12-18(13-26-23)17-11-20-21(15-28-24(20)27-14-17)16-7-3-1-4-8-16/h1-15,29H,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human PI3K p110gamma expressed in baculovirus expression system using PIP2/PS as substrate after 1 h... |
ACS Med Chem Lett 8: 875-880 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00222
BindingDB Entry DOI: 10.7270/Q2CR5WZT |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50267750

(CHEMBL1078685)Show InChI InChI=1S/C13H8N6/c14-8-11-7-10(3-6-16-11)13-17-12(18-19-13)9-1-4-15-5-2-9/h1-7H,(H,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
China Medical University
Curated by ChEMBL
| Assay Description
Antagonistic activity against the P2X7 ion channel |
Eur J Med Chem 141: 362-372 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.051
BindingDB Entry DOI: 10.7270/Q2FJ2K90 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data