 Found 306 hits with Last Name = 'niu' and Initial = 'h'
Found 306 hits with Last Name = 'niu' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127441
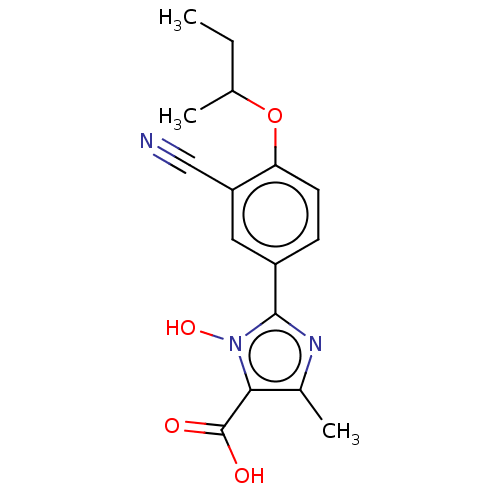
(CHEMBL3628191)Show InChI InChI=1S/C16H17N3O4/c1-4-9(2)23-13-6-5-11(7-12(13)8-17)15-18-10(3)14(16(20)21)19(15)22/h5-7,9,22H,4H2,1-3H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127486

(CHEMBL3628190)Show InChI InChI=1S/C16H17N3O4/c1-3-4-7-23-13-6-5-11(8-12(13)9-17)15-18-10(2)14(16(20)21)19(15)22/h5-6,8,22H,3-4,7H2,1-2H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50550025
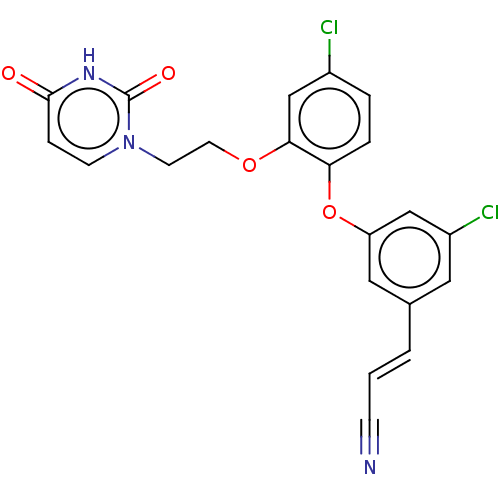
(CHEMBL1923490)Show SMILES Clc1ccc(Oc2cc(Cl)cc(\C=C\C#N)c2)c(OCCn2ccc(=O)[nH]c2=O)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant wild type HIV-1 reverse transcriptase using poly(rA)350/oligo(dT)16 as template/primer preincubated for 1 hr followed by su... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00612
BindingDB Entry DOI: 10.7270/Q2ZC86HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127441

(CHEMBL3628191)Show InChI InChI=1S/C16H17N3O4/c1-4-9(2)23-13-6-5-11(7-12(13)8-17)15-18-10(3)14(16(20)21)19(15)22/h5-7,9,22H,4H2,1-3H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127486

(CHEMBL3628190)Show InChI InChI=1S/C16H17N3O4/c1-3-4-7-23-13-6-5-11(8-12(13)9-17)15-18-10(2)14(16(20)21)19(15)22/h5-6,8,22H,3-4,7H2,1-2H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608309
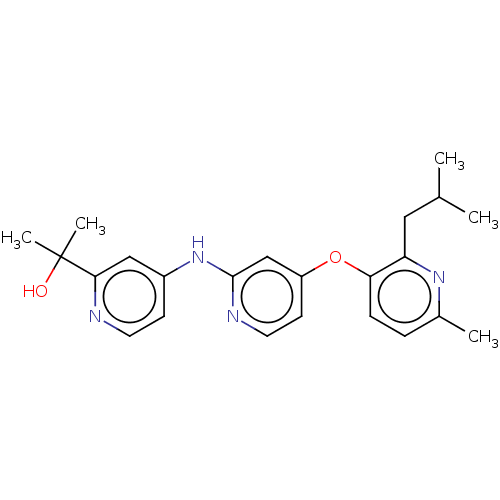
(US11691981, Compound 66)Show SMILES CC(C)Cc1nc(C)ccc1Oc1ccnc(Nc2ccnc(c2)C(C)(C)O)c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127487
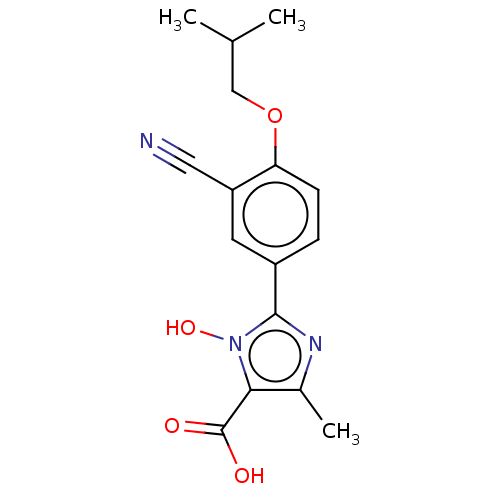
(CHEMBL3628192)Show InChI InChI=1S/C16H17N3O4/c1-9(2)8-23-13-5-4-11(6-12(13)7-17)15-18-10(3)14(16(20)21)19(15)22/h4-6,9,22H,8H2,1-3H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584925
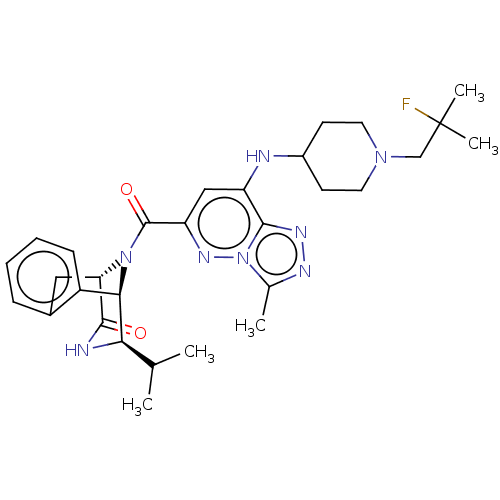
(CHEMBL5079885)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NC2CCN(CC(C)(C)F)CC2)c2nnc(C)n2n1 |r,THB:19:18:3.8.2:13.12.11| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (981 to 1108 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127487

(CHEMBL3628192)Show InChI InChI=1S/C16H17N3O4/c1-9(2)8-23-13-5-4-11(6-12(13)7-17)15-18-10(3)14(16(20)21)19(15)22/h4-6,9,22H,8H2,1-3H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584925

(CHEMBL5079885)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NC2CCN(CC(C)(C)F)CC2)c2nnc(C)n2n1 |r,THB:19:18:3.8.2:13.12.11| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (951 to 1132 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50320491
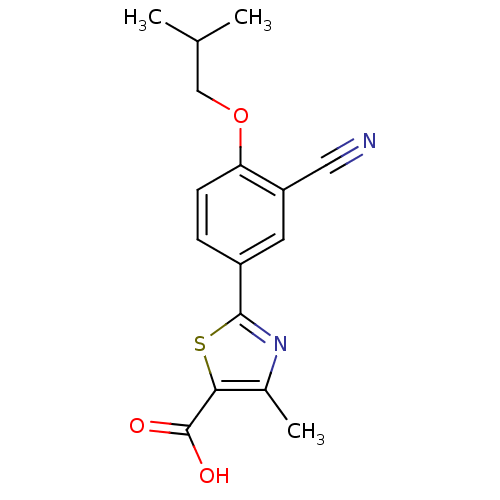
(2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE...)Show InChI InChI=1S/C16H16N2O3S/c1-9(2)8-21-13-5-4-11(6-12(13)7-17)15-18-10(3)14(22-15)16(19)20/h4-6,9H,8H2,1-3H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50320491

(2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE...)Show InChI InChI=1S/C16H16N2O3S/c1-9(2)8-21-13-5-4-11(6-12(13)7-17)15-18-10(3)14(22-15)16(19)20/h4-6,9H,8H2,1-3H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608284

(US11691981, Compound 41)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3cc(nn3-c3ccccn3)C(F)(F)F)ccn2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608255

(US11691981, Compound 17)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3cc(nn3-c3ccccc3)C(F)(F)F)ccn2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50365219
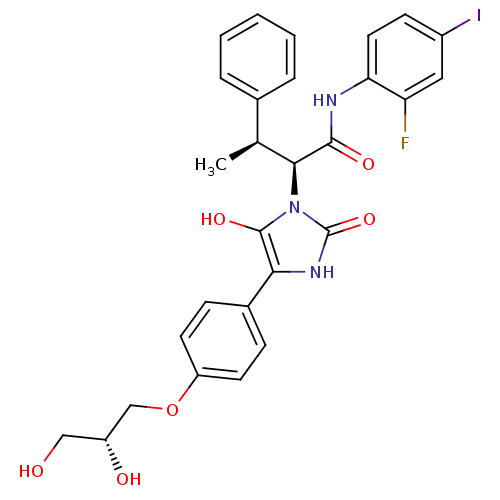
(CHEMBL1956073)Show SMILES C[C@H]([C@@H](C(=O)Nc1ccc(I)cc1F)n1c(O)c([nH]c1=O)-c1ccc(OC[C@H](O)CO)cc1)c1ccccc1 |r| Show InChI InChI=1S/C28H27FIN3O6/c1-16(17-5-3-2-4-6-17)25(26(36)31-23-12-9-19(30)13-22(23)29)33-27(37)24(32-28(33)38)18-7-10-21(11-8-18)39-15-20(35)14-34/h2-13,16,20,25,34-35,37H,14-15H2,1H3,(H,31,36)(H,32,38)/t16-,20+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 in human HT-29 cells assessed as inhibition of ERK phosphorylation by Western blot analysis |
Mol Cancer Ther 9: 134-44 (2010)
Article DOI: 10.1158/1535-7163.MCT-09-0601
BindingDB Entry DOI: 10.7270/Q2930TNW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608288
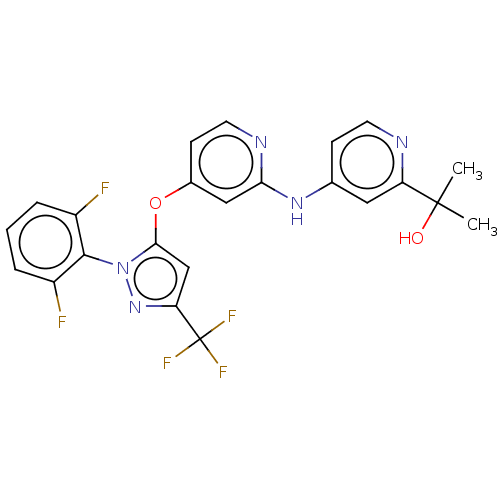
(US11691981, Compound 45)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3cc(nn3-c3c(F)cccc3F)C(F)(F)F)ccn2)ccn1 |(-8.08,-3.7,;-6.75,-4.47,;-6.75,-6.01,;-8.08,-5.24,;-5.42,-3.7,;-4.08,-4.47,;-2.75,-3.7,;-1.42,-4.47,;-.08,-3.7,;-.08,-2.16,;1.25,-1.39,;1.25,.15,;2.58,.92,;3.99,.29,;5.02,1.43,;4.25,2.77,;2.75,2.45,;1.6,3.48,;1.92,4.98,;3.39,5.46,;.78,6.01,;-.69,5.54,;-1.01,4.03,;.14,3,;-.18,1.5,;6.55,1.27,;7.46,2.52,;7.18,-.13,;8.08,1.11,;2.58,-2.16,;2.58,-3.7,;1.25,-4.47,;-2.75,-2.16,;-4.08,-1.39,;-5.42,-2.16,)| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608308
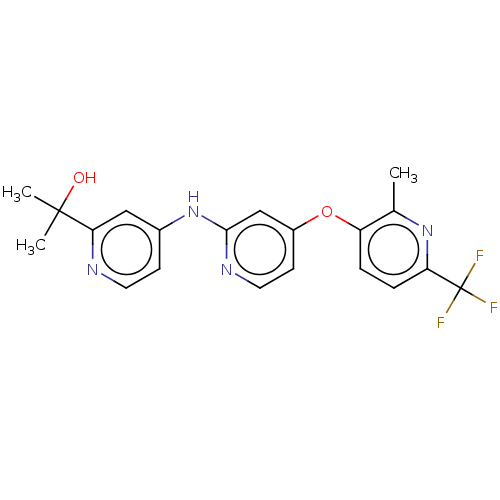
(US11691981, Compound 65)Show SMILES Cc1nc(ccc1Oc1ccnc(Nc2ccnc(c2)C(C)(C)O)c1)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584944
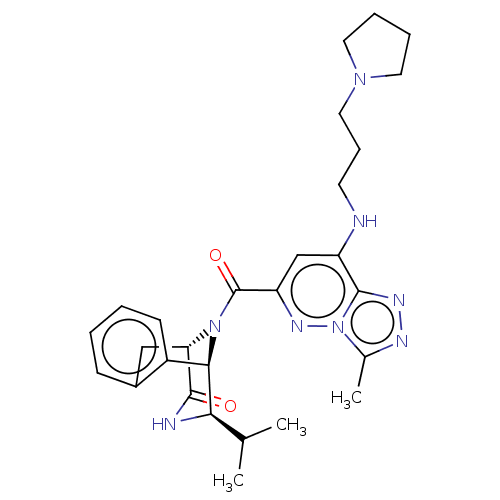
(CHEMBL5087319)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NCCCN2CCCC2)c2nnc(C)n2n1 |r,THB:19:18:11.12.13:3.8.2| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (951 to 1132 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608336

(US11691981, Compound 73)Show SMILES NS(=O)(=O)c1ccc(Nc2cc(Oc3cn(nc3C3CCOCC3)C3CC3)ccn2)cc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50550017
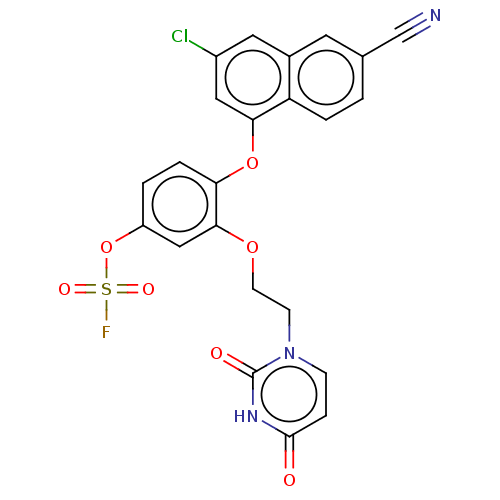
(CHEMBL4751058)Show SMILES FS(=O)(=O)Oc1ccc(Oc2cc(Cl)cc3cc(ccc23)C#N)c(OCCn2ccc(=O)[nH]c2=O)c1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Covalent inhibition of recombinant wild type HIV-1 reverse transcriptase using poly(rA)350/oligo(dT)16 as template/primer preincubated followed by su... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00612
BindingDB Entry DOI: 10.7270/Q2ZC86HB |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584944

(CHEMBL5087319)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NCCCN2CCCC2)c2nnc(C)n2n1 |r,THB:19:18:11.12.13:3.8.2| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (981 to 1108 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50564603

(CHEMBL4777294 | US11691981, Compound 77)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3cn(nc3C3CCC(F)(F)CC3)C3CC3)ccn2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608337

(US11691981, Compound 74)Show SMILES C[C@H]1CC(C[C@@H](C)O1)c1nn(cc1Oc1ccnc(Nc2ccnc(c2)C(C)(C)O)c1)C1CC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608296

(US11691981, Compound 53)Show SMILES CC(C)(O)c1ccc(Nc2cc(Oc3cc(nn3-c3c(F)cccc3F)C(F)(F)F)ccn2)cn1 |(-8.08,-2.93,;-6.75,-2.16,;-6.75,-.62,;-8.08,-1.39,;-5.42,-2.93,;-4.08,-2.16,;-2.75,-2.93,;-2.75,-4.47,;-1.42,-5.24,;-.08,-4.47,;-.08,-2.93,;1.25,-2.16,;1.25,-.62,;2.58,.15,;3.99,-.48,;5.02,.66,;4.25,2,;2.75,1.68,;1.6,2.71,;1.92,4.21,;3.39,4.69,;.78,5.24,;-.69,4.77,;-1.01,3.26,;.14,2.23,;-.18,.73,;6.55,.5,;7.46,1.75,;7.18,-.9,;8.08,.34,;2.58,-2.93,;2.58,-4.47,;1.25,-5.24,;-4.08,-5.24,;-5.42,-4.47,)| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608281

(US11691981, Compound 38)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3cc(nn3-c3ccccc3F)C(F)(F)F)ccn2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608271

(US11691981, Compound 29)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3cc(nn3[C@H]3C[C@@H]4CC[C@H](C3)O4)C3CC3)ccn2)ccn1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584945
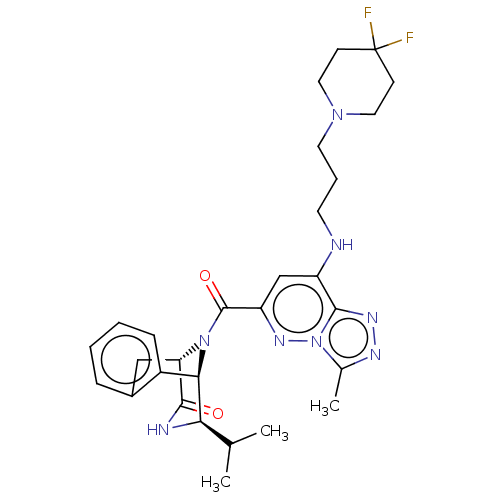
(CHEMBL5093862)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NCCCN2CCC(F)(F)CC2)c2nnc(C)n2n1 |r,THB:19:18:11.12.13:3.8.2| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (981 to 1108 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584945

(CHEMBL5093862)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NCCCN2CCC(F)(F)CC2)c2nnc(C)n2n1 |r,THB:19:18:11.12.13:3.8.2| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (951 to 1132 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584946
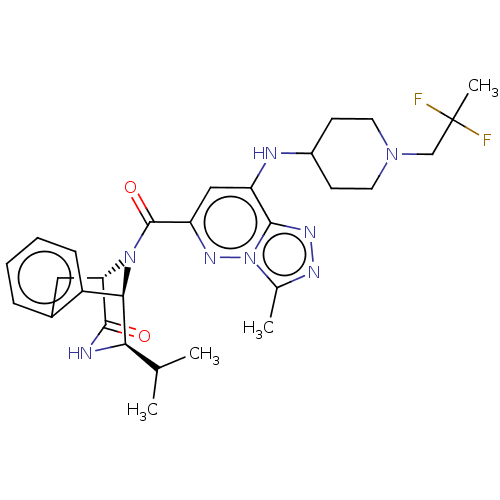
(CHEMBL5090369)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NC2CCN(CC(C)(F)F)CC2)c2nnc(C)n2n1 |r,THB:19:18:11.12.13:3.8.2| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (981 to 1108 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608300

(US11691981, Compound 57)Show SMILES Cc1ccc(Oc2ccnc(Nc3ccnc(c3)C(C)(C)O)c2)c(C)n1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608295
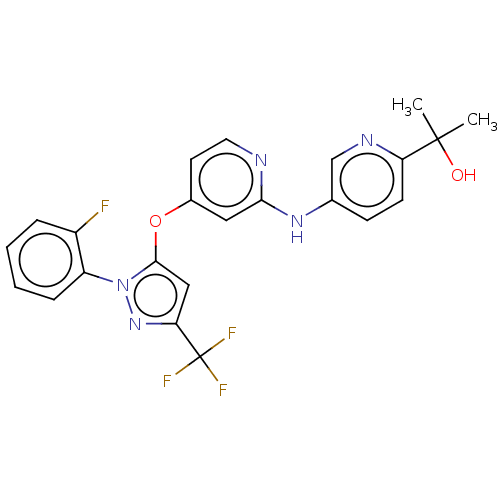
(US11691981, Compound 52)Show SMILES CC(C)(O)c1ccc(Nc2cc(Oc3cc(nn3-c3ccccc3F)C(F)(F)F)ccn2)cn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608341

(US11691981, Compound 78)Show SMILES CC1(C)OC(=N)c2ccc(Nc3cc(Oc4cn(nc4C4CCOCC4)C4CC4)ccn3)cc12 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50564620

(Ly-3200882 | Ly3200882 | US11691981, Compound A)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3cn(nc3C3CCOCC3)C3CC3)ccn2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584946

(CHEMBL5090369)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NC2CCN(CC(C)(F)F)CC2)c2nnc(C)n2n1 |r,THB:19:18:11.12.13:3.8.2| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (951 to 1132 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50365219

(CHEMBL1956073)Show SMILES C[C@H]([C@@H](C(=O)Nc1ccc(I)cc1F)n1c(O)c([nH]c1=O)-c1ccc(OC[C@H](O)CO)cc1)c1ccccc1 |r| Show InChI InChI=1S/C28H27FIN3O6/c1-16(17-5-3-2-4-6-17)25(26(36)31-23-12-9-19(30)13-22(23)29)33-27(37)24(32-28(33)38)18-7-10-21(11-8-18)39-15-20(35)14-34/h2-13,16,20,25,34-35,37H,14-15H2,1H3,(H,31,36)(H,32,38)/t16-,20+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 in human LOX cells assessed as inhibition of ERK phosphorylation by Western blot analysis |
Mol Cancer Ther 9: 134-44 (2010)
Article DOI: 10.1158/1535-7163.MCT-09-0601
BindingDB Entry DOI: 10.7270/Q2930TNW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608247

(US11691981, Compound 11)Show SMILES CC(C)(O)c1ccc(Nc2cc(Oc3cc(nn3C3CCOCC3)C(F)(F)F)ccn2)cn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50365219

(CHEMBL1956073)Show SMILES C[C@H]([C@@H](C(=O)Nc1ccc(I)cc1F)n1c(O)c([nH]c1=O)-c1ccc(OC[C@H](O)CO)cc1)c1ccccc1 |r| Show InChI InChI=1S/C28H27FIN3O6/c1-16(17-5-3-2-4-6-17)25(26(36)31-23-12-9-19(30)13-22(23)29)33-27(37)24(32-28(33)38)18-7-10-21(11-8-18)39-15-20(35)14-34/h2-13,16,20,25,34-35,37H,14-15H2,1H3,(H,31,36)(H,32,38)/t16-,20+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 |
Mol Cancer Ther 9: 134-44 (2010)
Article DOI: 10.1158/1535-7163.MCT-09-0601
BindingDB Entry DOI: 10.7270/Q2930TNW |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608314

(US11691981, Compound 71)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3c4CCCn4nc3C3CCOCC3)ccn2)ccn1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608248
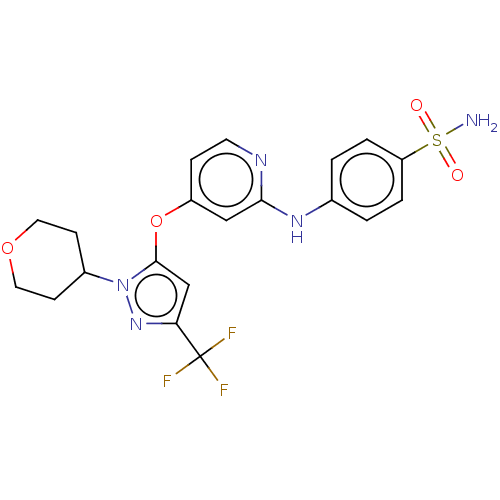
(US11691981, Compound 12)Show SMILES NS(=O)(=O)c1ccc(Nc2cc(Oc3cc(nn3C3CCOCC3)C(F)(F)F)ccn2)cc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608342

(US11691981, Compound 79)Show SMILES CC1(C)OC(=O)c2ccc(Nc3cc(Oc4cn(nc4C4CCOCC4)C4CC4)ccn3)cc12 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608305

(US11691981, Compound 62)Show SMILES Cc1ccc(Oc2ccnc(Nc3ccc(nc3)C(C)(C)O)c2)c(C)n1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127489
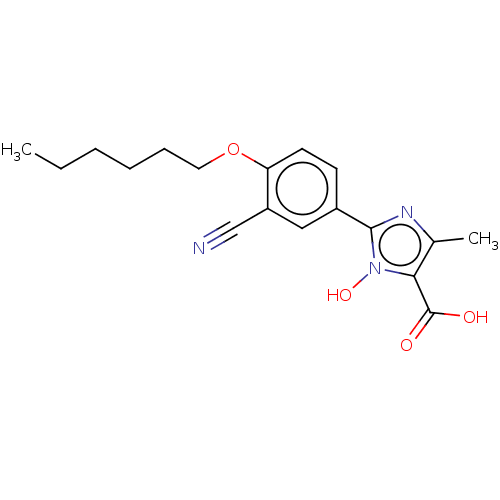
(CHEMBL3628194)Show InChI InChI=1S/C18H21N3O4/c1-3-4-5-6-9-25-15-8-7-13(10-14(15)11-19)17-20-12(2)16(18(22)23)21(17)24/h7-8,10,24H,3-6,9H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127488
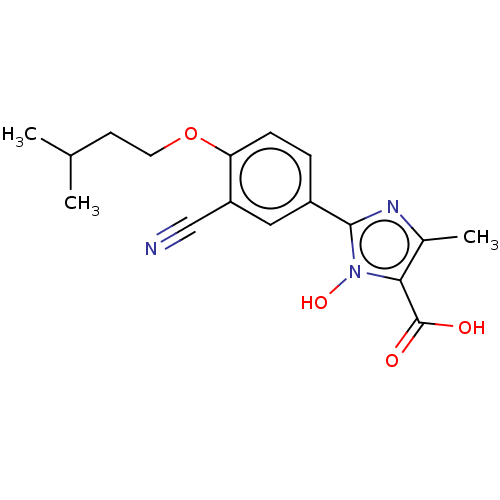
(CHEMBL3628193)Show InChI InChI=1S/C17H19N3O4/c1-10(2)6-7-24-14-5-4-12(8-13(14)9-18)16-19-11(3)15(17(21)22)20(16)23/h4-5,8,10,23H,6-7H2,1-3H3,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608292

(US11691981, Compound 49)Show SMILES CC(C)(O)c1cc(Nc2cc(Oc3cc(nn3[C@H]3C[C@@H]4CC[C@H](C3)O4)C(F)(F)F)ccn2)ccn1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608302

(US11691981, Compound 59)Show SMILES Cc1ccc(Oc2ccnc(Nc3ccc(cc3)S(N)(=O)=O)c2)c(n1)C1CCOCC1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584941
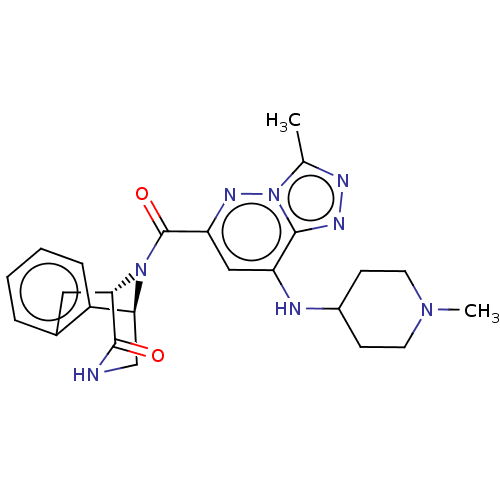
(CHEMBL5077167)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])(CNC1=O)N2C(=O)c1cc(NC2CCN(C)CC2)c2nnc(C)n2n1 |r,THB:16:15:11.12.13:3.8.2| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (981 to 1108 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127488

(CHEMBL3628193)Show InChI InChI=1S/C17H19N3O4/c1-10(2)6-7-24-14-5-4-12(8-13(14)9-18)16-19-11(3)15(17(21)22)20(16)23/h4-5,8,10,23H,6-7H2,1-3H3,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Bos taurus (Bovine)) | BDBM50127489

(CHEMBL3628194)Show InChI InChI=1S/C18H21N3O4/c1-3-4-5-6-9-25-15-8-7-13(10-14(15)11-19)17-20-12(2)16(18(22)23)21(17)24/h7-8,10,24H,3-6,9H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of bovine XO using xanthine as substrate after 120 mins by spectrophotometric analysis |
Eur J Med Chem 103: 343-53 (2015)
Article DOI: 10.1016/j.ejmech.2015.08.056
BindingDB Entry DOI: 10.7270/Q2X3509Z |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608307

(US11691981, Compound 64)Show SMILES COc1ccc(Oc2ccnc(Nc3ccnc(c3)C(C)(C)O)c2)c(C)n1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM608254

(US11691981, Compound 16)Show SMILES CC(C)(C)n1nc(cc1Oc1ccnc(Nc2ccnc(c2)C(C)(C)O)c1)C(F)(F)F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2Z323R6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data