

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50523555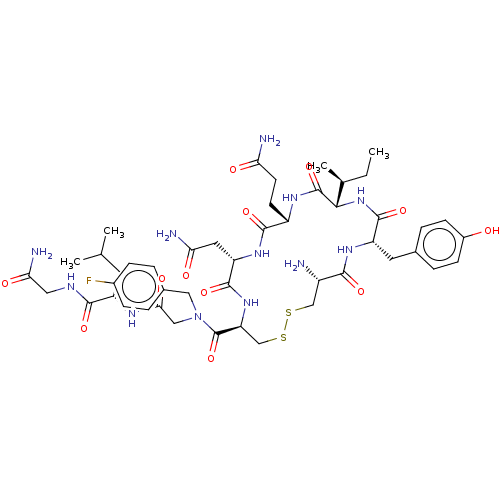 (CHEMBL4474284) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50205990 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50523556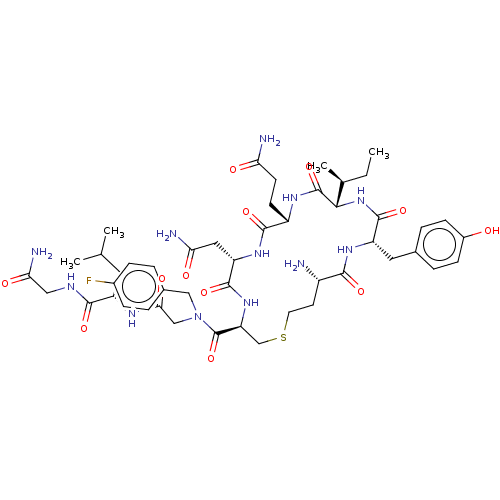 (CHEMBL4458988) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044686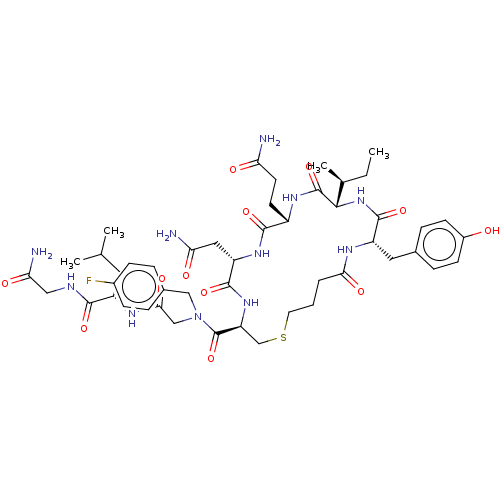 (CHEMBL3354594) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50523557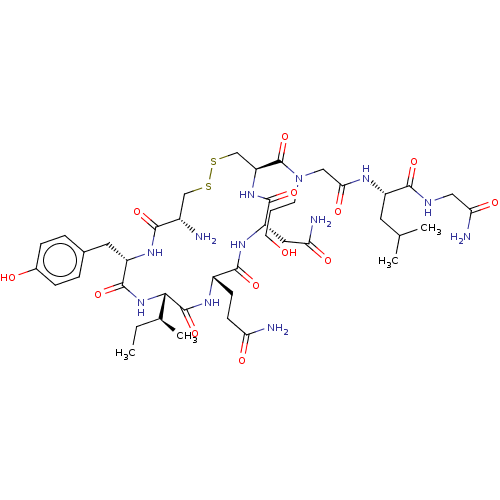 (CHEMBL4583231) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044742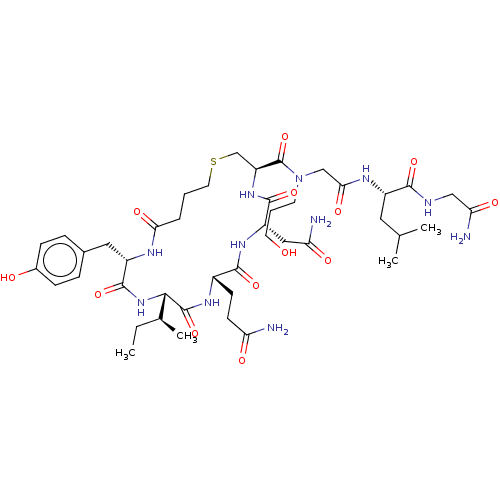 (CHEMBL3354579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044677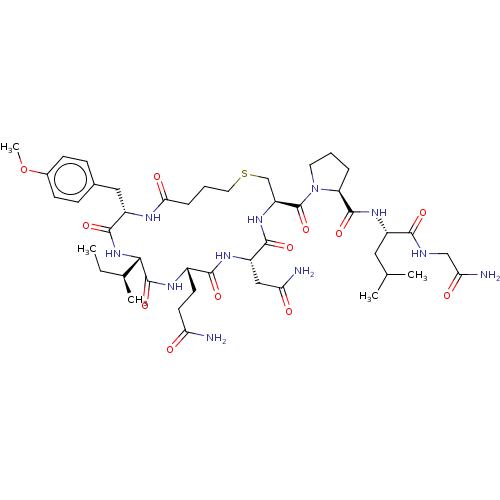 (CHEBI:59204 | Carbetocin) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50044777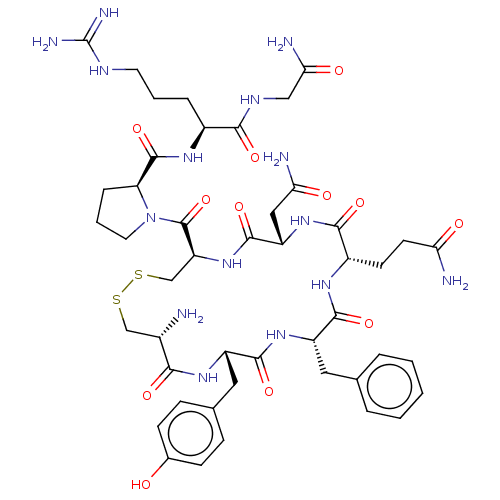 (Arginine Vasopressin | Beta-Hypophamine | CHEBI:34...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50523554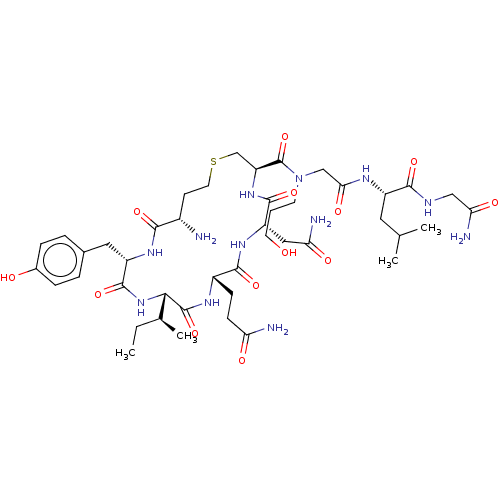 (CHEMBL4467042) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University and Department of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hr | J Med Chem 62: 3297-3310 (2019) Article DOI: 10.1021/acs.jmedchem.8b01691 BindingDB Entry DOI: 10.7270/Q2GF0XXQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50608178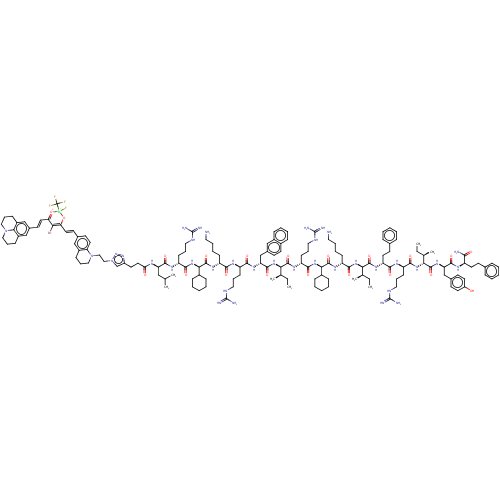 (CHEMBL5268044) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50608179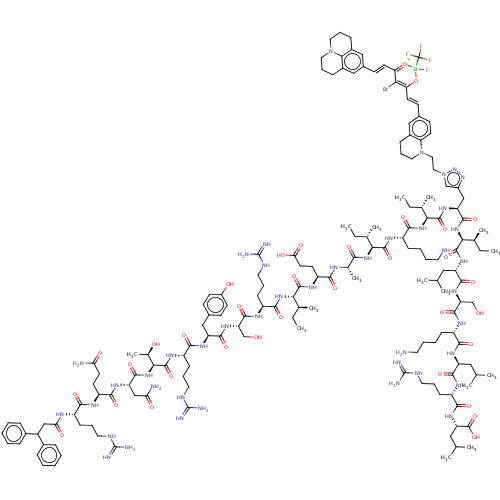 (CHEMBL5285634) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50585028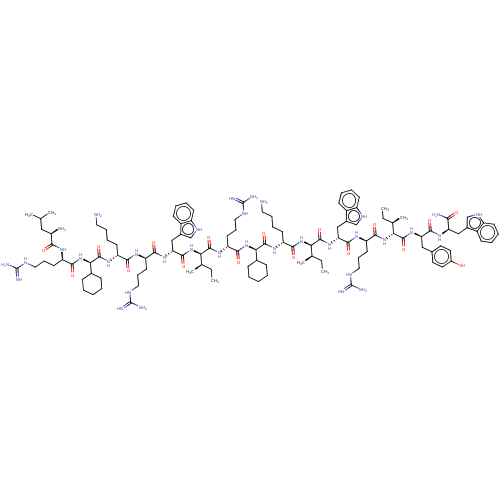 (CHEMBL5078185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50585028 (CHEMBL5078185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth/differentiation factor 8 (Homo sapiens (Human)) | BDBM50585029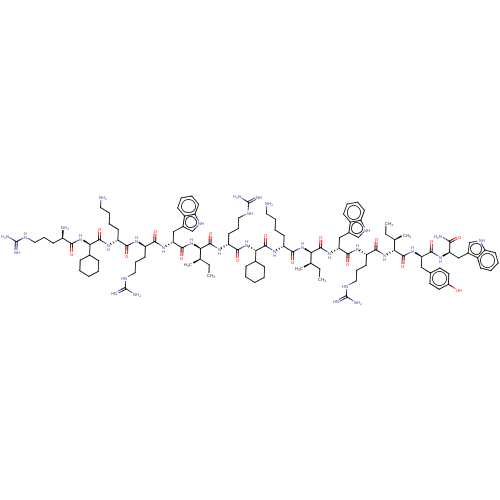 (CHEMBL5092917) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of myostatin (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual l... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transforming growth factor beta-1 proprotein (Homo sapiens (Human)) | BDBM50585028 (CHEMBL5078185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGF beta 1 (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145552 (2,2-Dimethyl-thiopropionic acid S-(4,5-dichloro-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369597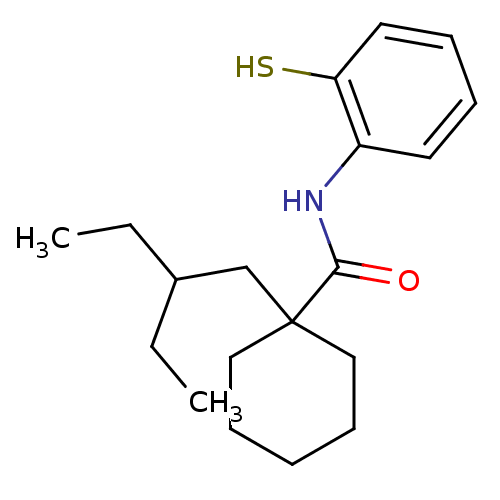 (CHEMBL117102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369594 (CHEMBL332976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50092197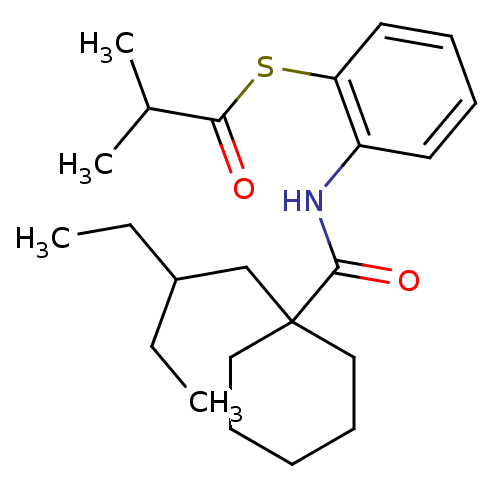 (CHEMBL313006 | S-2-(1-(2-ethylbutyl)cyclohexanecar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369592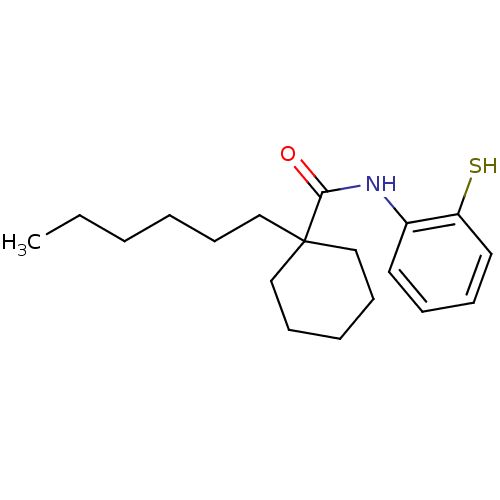 (CHEMBL120839) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50092197 (CHEMBL313006 | S-2-(1-(2-ethylbutyl)cyclohexanecar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369617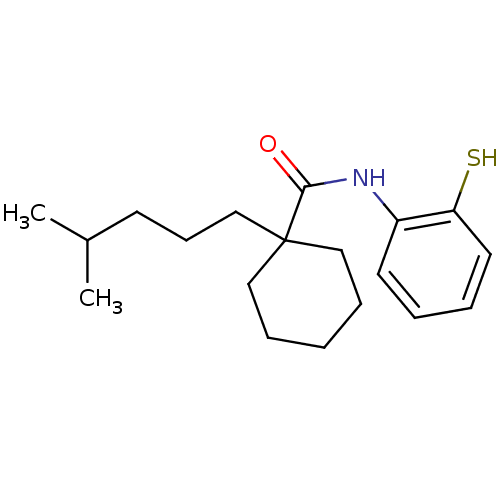 (CHEMBL120054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transforming growth factor beta-1 proprotein (Homo sapiens (Human)) | BDBM50525413 (CHEMBL4448117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TGF beta 1 (unknown origin) expressed in HEK293 cells transfected with Smad2/3 responsive reporter plasmid incubated for 4 hrs by dual ... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00705 BindingDB Entry DOI: 10.7270/Q28S4TT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369607 (CHEMBL121096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369615 (CHEMBL118867) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369612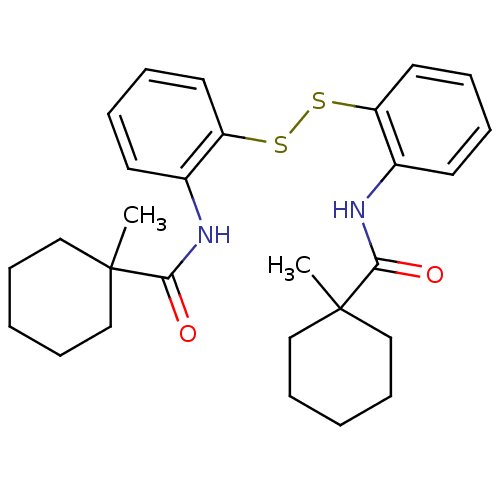 (CHEMBL118757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369604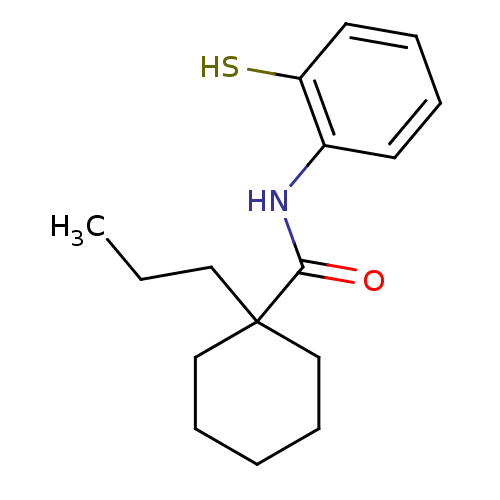 (CHEMBL265812) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369611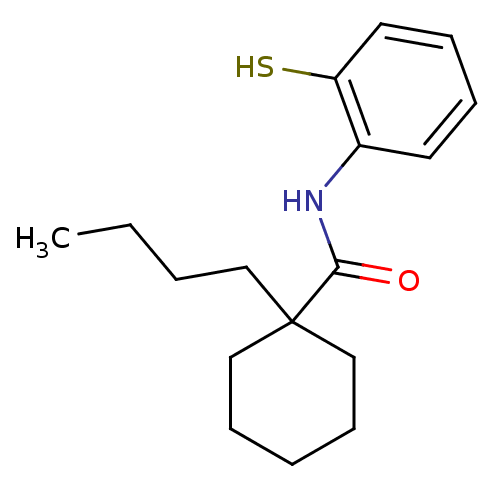 (CHEMBL331175) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145558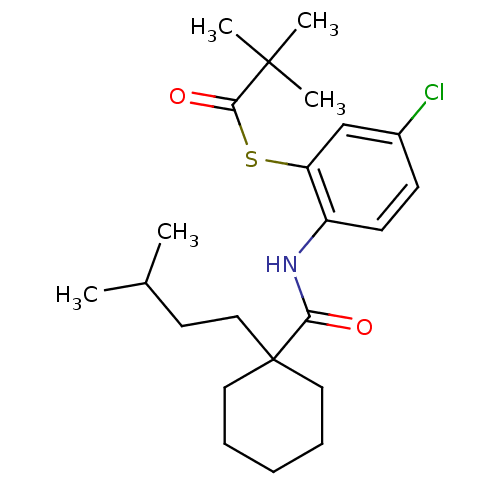 (2,2-Dimethyl-thiopropionic acid S-(5-chloro-2-{[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369593 (CHEMBL118472) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145541 (2,2-Dimethyl-thiopropionic acid S-(4-chloro-2-{[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145551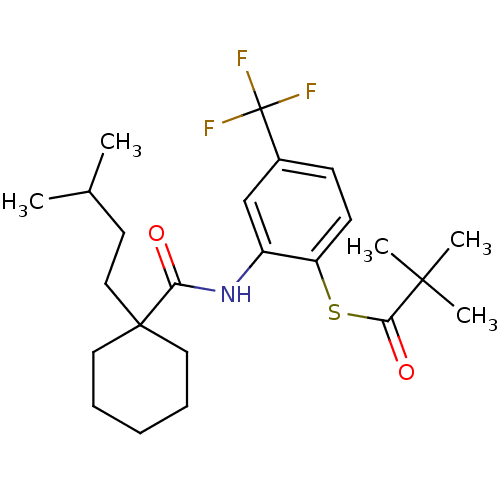 (2,2-Dimethyl-thiopropionic acid S-(2-{[1-(3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369601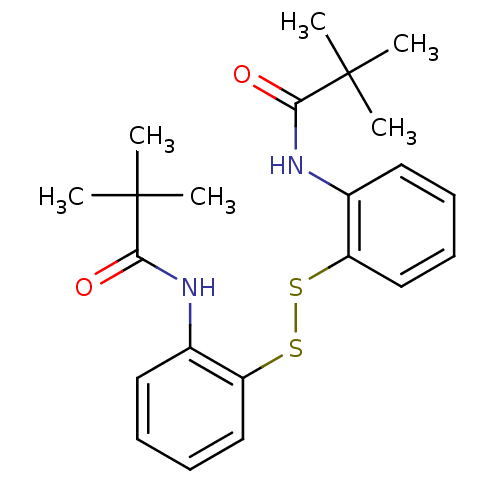 (CHEMBL332163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369599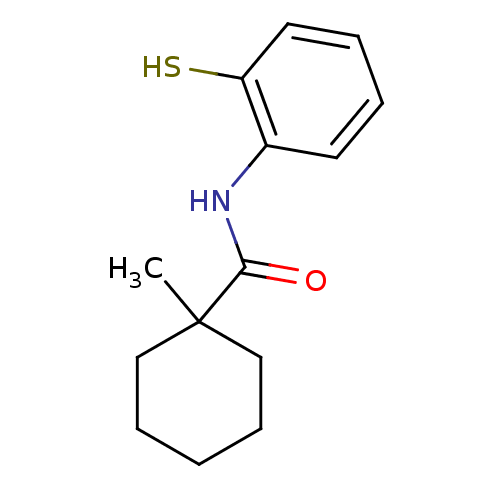 (CHEMBL120059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369600 (CHEMBL331064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369595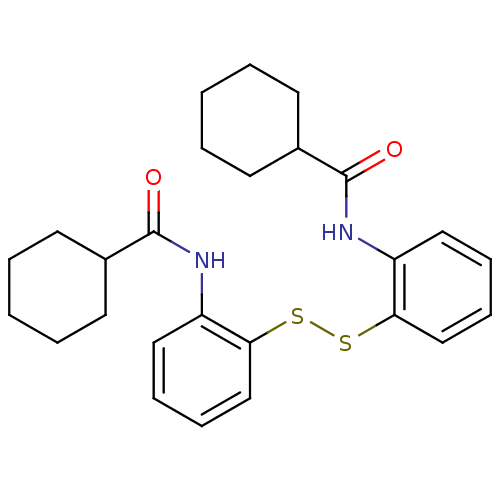 (CHEMBL333203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369608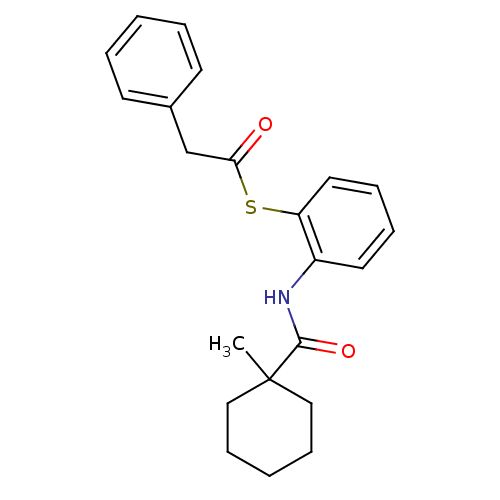 (CHEMBL339196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145557 (2,2-Dimethyl-thiopropionic acid S-(4-cyano-2-{[1-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369598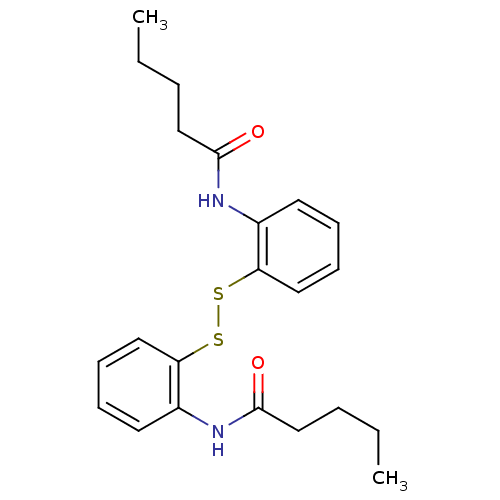 (CHEMBL118624) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145546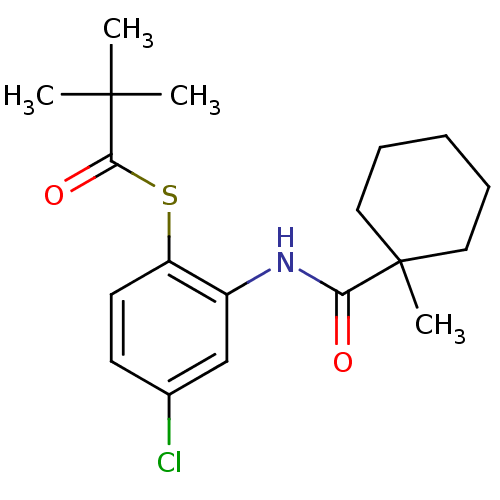 (2,2-Dimethyl-thiopropionic acid S-{4-chloro-2-[(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145549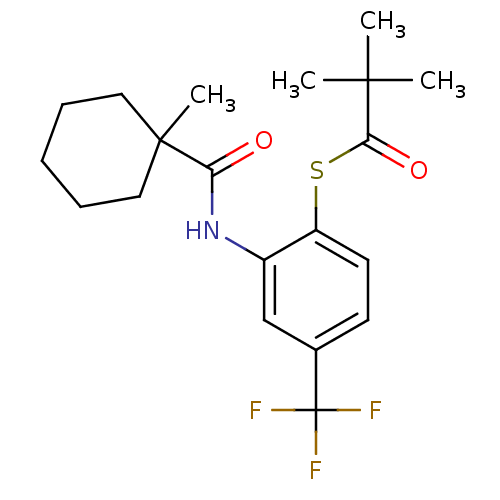 (2,2-Dimethyl-thiopropionic acid S-{2-[(1-methyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369613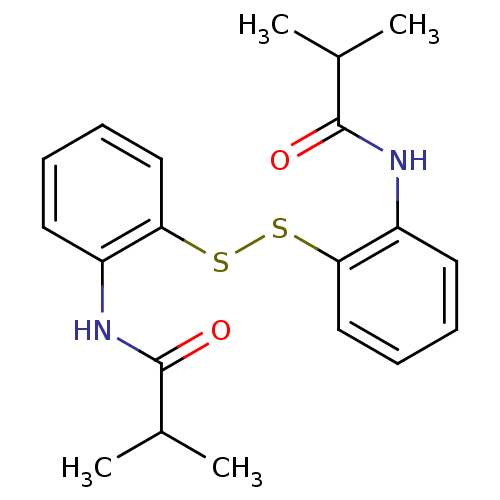 (CHEMBL116713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369602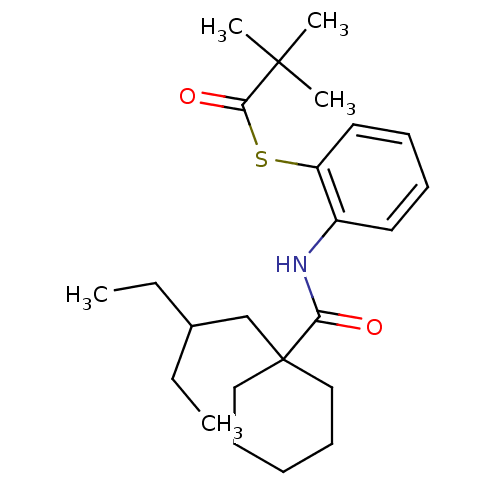 (CHEMBL120087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145550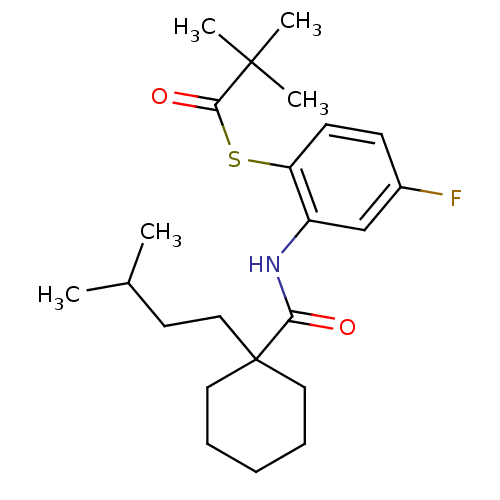 (2,2-Dimethyl-thiopropionic acid S-(4-fluoro-2-{[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145547 (2,2-Dimethyl-thiopropionic acid S-(2-{[1-(3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145556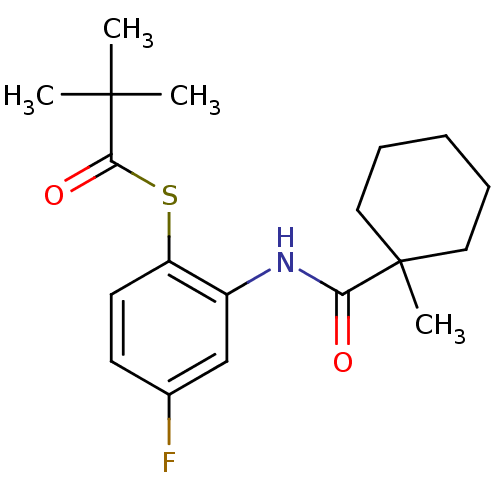 (2,2-Dimethyl-thiopropionic acid S-{4-fluoro-2-[(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145544 (2,2-Dimethyl-thiopropionic acid S-{2-[(1-methyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50145548 (2,2-Dimethyl-thiopropionic acid S-{5-fluoro-2-[(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of CETP activity was assessed by measuring the rate of [3H]- cholesteryl ester transfer from HDL to apoprotein B-containing lipop... | Bioorg Med Chem Lett 14: 2589-91 (2004) Article DOI: 10.1016/j.bmcl.2004.02.071 BindingDB Entry DOI: 10.7270/Q2VQ324C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50369603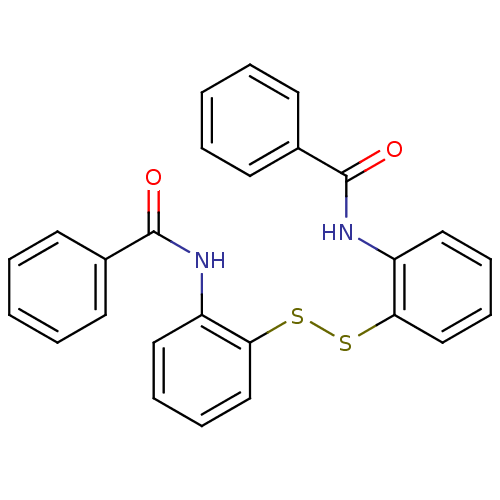 (CHEMBL118148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description In vitro concentration required to inhibit 50% of cholesteryl ester transfer protein mediated cholesteryl ester transfer from HDL to VLDL and LDL in ... | J Med Chem 43: 3566-72 (2000) BindingDB Entry DOI: 10.7270/Q22J6CK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 91 total ) | Next | Last >> |