 Found 159 hits with Last Name = 'omar' and Initial = 'ha'
Found 159 hits with Last Name = 'omar' and Initial = 'ha' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Flt4 (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117930
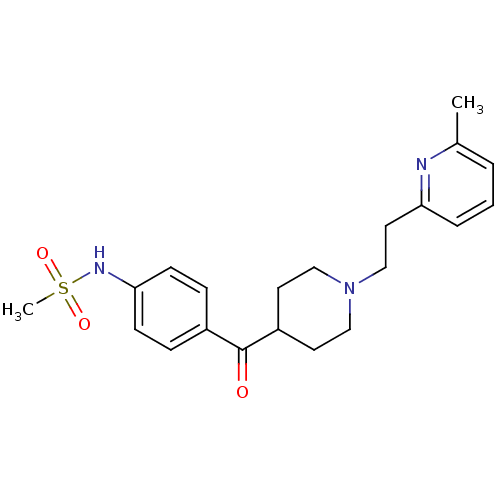
((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...)Show SMILES Cc1cccc(CCN2CCC(CC2)C(=O)c2ccc(NS(C)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H27N3O3S/c1-16-4-3-5-19(22-16)12-15-24-13-10-18(11-14-24)21(25)17-6-8-20(9-7-17)23-28(2,26)27/h3-9,18,23H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full-length GST-tagged human B-RAF V600E mutant (417 to 766 residues) expressed in Baculovirus infected Sf9 cells using N-t... |
Eur J Med Chem 150: 567-578 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.001
BindingDB Entry DOI: 10.7270/Q26D5WNS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5446

(CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...)Show InChI InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Inhibition of His-6 tagged recombinant EGFR cytoplasmic domain (645 to 1186 residues) (unknown origin) expressed in Baculovirus infected Sf9 cells by... |
Eur J Med Chem 150: 567-578 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.001
BindingDB Entry DOI: 10.7270/Q26D5WNS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KDR (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50562672
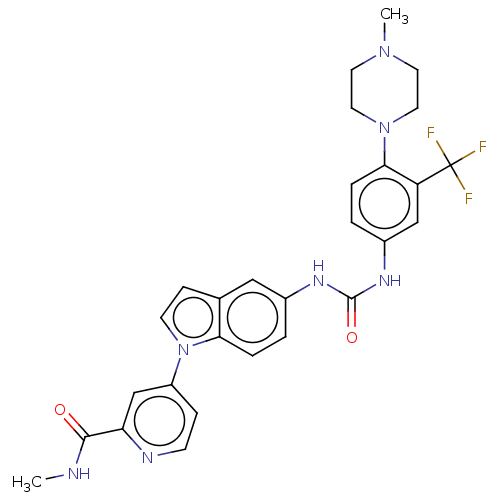
(CHEMBL4797840)Show SMILES CNC(=O)c1cc(ccn1)-n1ccc2cc(NC(=O)Nc3ccc(N4CCN(C)CC4)c(c3)C(F)(F)F)ccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Flt4 (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592452

(CHEMBL5191468)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCCNC(=O)Nc2ccc(F)cc2)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592452

(CHEMBL5191468)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCCNC(=O)Nc2ccc(F)cc2)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592449
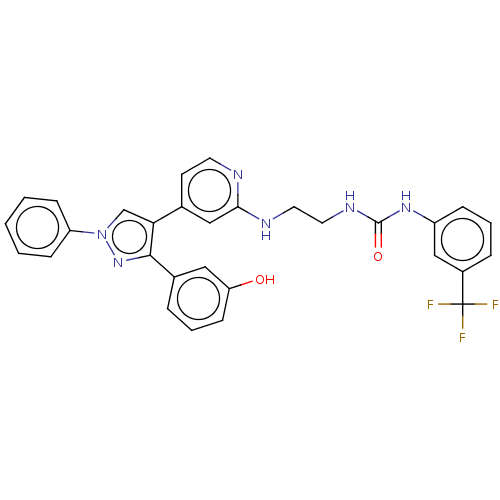
(CHEMBL5201911)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCNC(=O)Nc2cccc(c2)C(F)(F)F)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592449

(CHEMBL5201911)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCNC(=O)Nc2cccc(c2)C(F)(F)F)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261639

(CHEMBL4076689)Show SMILES O=C(Nc1ccccc1)c1c(N=Cc2cccnc2)c(C#N)c2CCCn12 Show InChI InChI=1S/C21H17N5O/c22-12-17-18-9-5-11-26(18)20(21(27)25-16-7-2-1-3-8-16)19(17)24-14-15-6-4-10-23-13-15/h1-4,6-8,10,13-14H,5,9,11H2,(H,25,27)/b24-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261633

(CHEMBL4079032)Show SMILES Cc1ccc(NC(=O)c2c(N=Cc3ccc4ccccc4c3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C27H22N4O/c1-18-8-12-22(13-9-18)30-27(32)26-25(23(16-28)24-7-4-14-31(24)26)29-17-19-10-11-20-5-2-3-6-21(20)15-19/h2-3,5-6,8-13,15,17H,4,7,14H2,1H3,(H,30,32)/b29-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261638
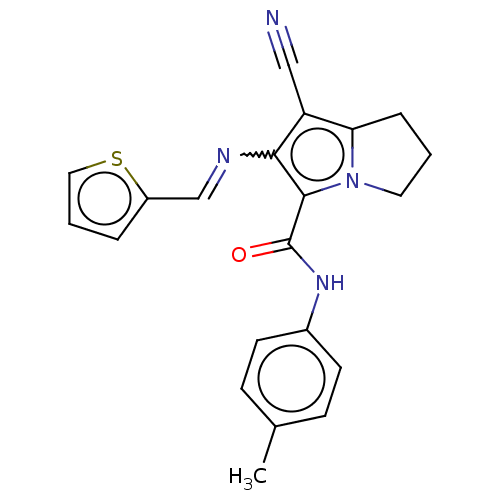
(CHEMBL4073779)Show SMILES Cc1ccc(NC(=O)c2c(N=Cc3cccs3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C21H18N4OS/c1-14-6-8-15(9-7-14)24-21(26)20-19(23-13-16-4-3-11-27-16)17(12-22)18-5-2-10-25(18)20/h3-4,6-9,11,13H,2,5,10H2,1H3,(H,24,26)/b23-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261640

(CHEMBL4096865)Show SMILES Cc1ccc(NC(=O)c2c(N=Cc3cccnc3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C22H19N5O/c1-15-6-8-17(9-7-15)26-22(28)21-20(25-14-16-4-2-10-24-13-16)18(12-23)19-5-3-11-27(19)21/h2,4,6-10,13-14H,3,5,11H2,1H3,(H,26,28)/b25-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261634
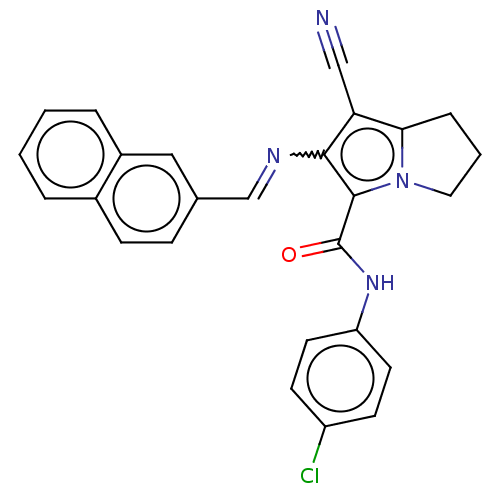
(CHEMBL4097045)Show SMILES Clc1ccc(NC(=O)c2c(N=Cc3ccc4ccccc4c3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C26H19ClN4O/c27-20-9-11-21(12-10-20)30-26(32)25-24(22(15-28)23-6-3-13-31(23)25)29-16-17-7-8-18-4-1-2-5-19(18)14-17/h1-2,4-5,7-12,14,16H,3,6,13H2,(H,30,32)/b29-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50562672

(CHEMBL4797840)Show SMILES CNC(=O)c1cc(ccn1)-n1ccc2cc(NC(=O)Nc3ccc(N4CCN(C)CC4)c(c3)C(F)(F)F)ccc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of KDR (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261643

(CHEMBL4071061)Show SMILES Cc1ccc(NC(=O)c2c(N=Cc3ccco3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C21H18N4O2/c1-14-6-8-15(9-7-14)24-21(26)20-19(23-13-16-4-3-11-27-16)17(12-22)18-5-2-10-25(18)20/h3-4,6-9,11,13H,2,5,10H2,1H3,(H,24,26)/b23-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592447
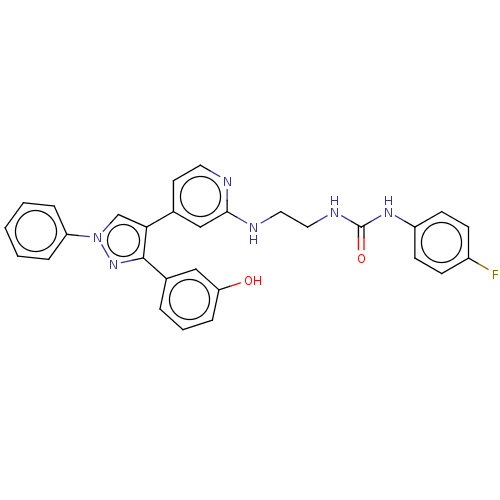
(CHEMBL5196004)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCNC(=O)Nc2ccc(F)cc2)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592447

(CHEMBL5196004)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCNC(=O)Nc2ccc(F)cc2)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 191 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Homo sapiens (Human)) | BDBM50504119
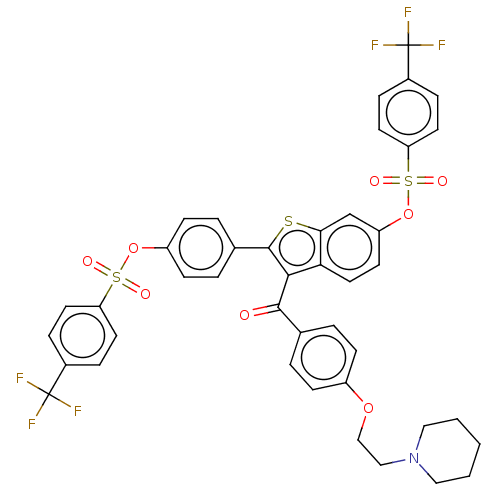
(CHEMBL4540180)Show SMILES FC(F)(F)c1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1sc2cc(OS(=O)(=O)c3ccc(cc3)C(F)(F)F)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C42H33F6NO8S3/c43-41(44,45)29-8-17-34(18-9-29)59(51,52)56-32-14-6-28(7-15-32)40-38(39(50)27-4-12-31(13-5-27)55-25-24-49-22-2-1-3-23-49)36-21-16-33(26-37(36)58-40)57-60(53,54)35-19-10-30(11-20-35)42(46,47)48/h4-21,26H,1-3,22-25H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human NPP3 expressed in COS-7 cell membranes assessed as reduction in p-nitrophenol production using pNP-TMP as substrate preincubated ... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.063
BindingDB Entry DOI: 10.7270/Q2B85CD9 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50504116
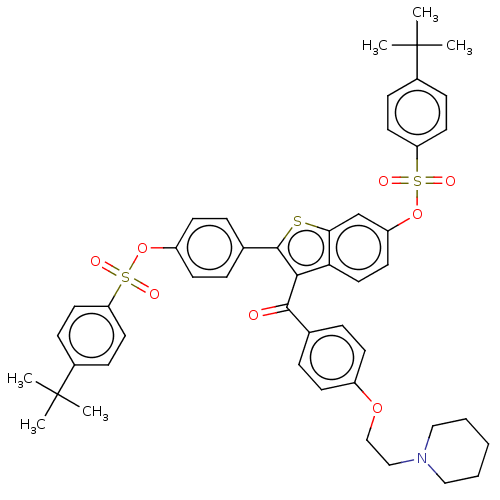
(CHEMBL4473374)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1sc2cc(OS(=O)(=O)c3ccc(cc3)C(C)(C)C)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C48H51NO8S3/c1-47(2,3)35-14-23-40(24-15-35)59(51,52)56-38-20-12-34(13-21-38)46-44(45(50)33-10-18-37(19-11-33)55-31-30-49-28-8-7-9-29-49)42-27-22-39(32-43(42)58-46)57-60(53,54)41-25-16-36(17-26-41)48(4,5)6/h10-27,32H,7-9,28-31H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS-7 cell membranes assessed as reduction in p-nitrophenol production using pNP-TMP as substrate preincubated ... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.063
BindingDB Entry DOI: 10.7270/Q2B85CD9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50207432

(CHEMBL3909479)Show SMILES COc1ccc(cc1)-c1nc(NCC(O)=O)sc1-c1ccc(OC)cc1 Show InChI InChI=1S/C19H18N2O4S/c1-24-14-7-3-12(4-8-14)17-18(13-5-9-15(25-2)10-6-13)26-19(21-17)20-11-16(22)23/h3-10H,11H2,1-2H3,(H,20,21)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... |
Bioorg Med Chem 25: 665-676 (2017)
Article DOI: 10.1016/j.bmc.2016.11.037
BindingDB Entry DOI: 10.7270/Q2542QKM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed... |
Bioorg Med Chem 25: 665-676 (2017)
Article DOI: 10.1016/j.bmc.2016.11.037
BindingDB Entry DOI: 10.7270/Q2542QKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261644
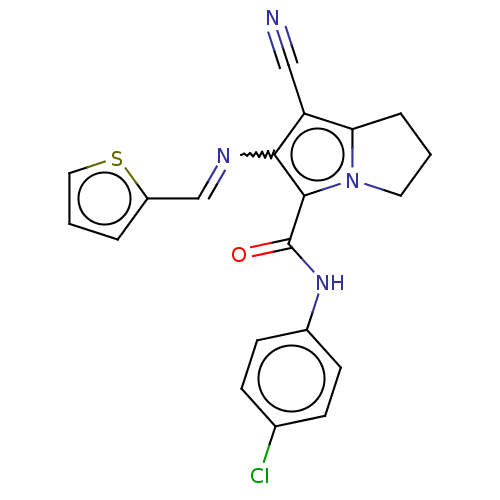
(CHEMBL4072244)Show SMILES Clc1ccc(NC(=O)c2c(N=Cc3cccs3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C20H15ClN4OS/c21-13-5-7-14(8-6-13)24-20(26)19-18(23-12-15-3-2-10-27-15)16(11-22)17-4-1-9-25(17)19/h2-3,5-8,10,12H,1,4,9H2,(H,24,26)/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592470

(CHEMBL5189795 | US20240002365, Compound CTx-029488...)Show SMILES CNC(=O)c1ccccc1Nc1nc(Nc2ccc(cc2)N2CCNCC2)ncc1Cl | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50207431
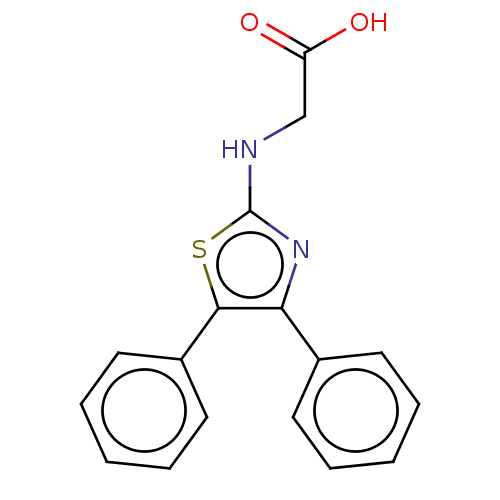
(CHEMBL3892572)Show InChI InChI=1S/C17H14N2O2S/c20-14(21)11-18-17-19-15(12-7-3-1-4-8-12)16(22-17)13-9-5-2-6-10-13/h1-10H,11H2,(H,18,19)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... |
Bioorg Med Chem 25: 665-676 (2017)
Article DOI: 10.1016/j.bmc.2016.11.037
BindingDB Entry DOI: 10.7270/Q2542QKM |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50504120

(CHEMBL4589775)Show SMILES COc1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1sc2cc(OS(=O)(=O)c3ccc(OC)cc3)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C42H39NO10S3/c1-49-31-14-19-36(20-15-31)55(45,46)52-34-12-8-30(9-13-34)42-40(41(44)29-6-10-33(11-7-29)51-27-26-43-24-4-3-5-25-43)38-23-18-35(28-39(38)54-42)53-56(47,48)37-21-16-32(50-2)17-22-37/h6-23,28H,3-5,24-27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS-7 cell membranes assessed as reduction in p-nitrophenol production using pNP-TMP as substrate preincubated ... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.063
BindingDB Entry DOI: 10.7270/Q2B85CD9 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 427 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDGFRalpha (unknown origin) in presence of [gamma33]-ATP by liquid scintillation counting analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113081
BindingDB Entry DOI: 10.7270/Q2B85CV4 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50504118

(CHEMBL4456605)Show SMILES Cc1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1sc2cc(OS(=O)(=O)c3ccc(C)cc3)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C42H39NO8S3/c1-29-6-19-36(20-7-29)53(45,46)50-34-16-12-32(13-17-34)42-40(41(44)31-10-14-33(15-11-31)49-27-26-43-24-4-3-5-25-43)38-23-18-35(28-39(38)52-42)51-54(47,48)37-21-8-30(2)9-22-37/h6-23,28H,3-5,24-27H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 expressed in COS-7 cell membranes assessed as reduction in p-nitrophenol production using pNP-TMP as substrate preincubated ... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.063
BindingDB Entry DOI: 10.7270/Q2B85CD9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261636

(CHEMBL4065185)Show SMILES Clc1ccc(NC(=O)c2c(N=Cc3ccco3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C20H15ClN4O2/c21-13-5-7-14(8-6-13)24-20(26)19-18(23-12-15-3-2-10-27-15)16(11-22)17-4-1-9-25(17)19/h2-3,5-8,10,12H,1,4,9H2,(H,24,26)/b23-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592454

(CHEMBL5205920)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCCNC(=O)Nc2cccc(c2)C(F)(F)F)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 474 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592454

(CHEMBL5205920)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCCNC(=O)Nc2cccc(c2)C(F)(F)F)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Homo sapiens (Human)) | BDBM50504124
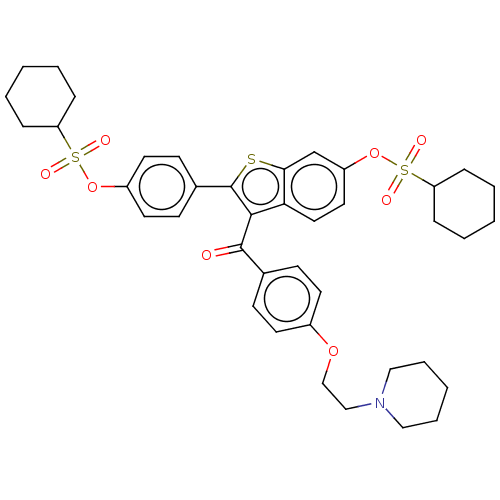
(CHEMBL4561757)Show SMILES O=C(c1c(sc2cc(OS(=O)(=O)C3CCCCC3)ccc12)-c1ccc(OS(=O)(=O)C2CCCCC2)cc1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C40H47NO8S3/c42-39(29-14-18-31(19-15-29)47-27-26-41-24-8-3-9-25-41)38-36-23-22-33(49-52(45,46)35-12-6-2-7-13-35)28-37(36)50-40(38)30-16-20-32(21-17-30)48-51(43,44)34-10-4-1-5-11-34/h14-23,28,34-35H,1-13,24-27H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human NPP3 expressed in COS-7 cell membranes assessed as reduction in p-nitrophenol production using pNP-TMP as substrate preincubated ... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.063
BindingDB Entry DOI: 10.7270/Q2B85CD9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261642

(CHEMBL4073566)Show SMILES O=C(Nc1ccccc1)c1c(N=Cc2ccc3ccccc3c2)c(C#N)c2CCCn12 Show InChI InChI=1S/C26H20N4O/c27-16-22-23-11-6-14-30(23)25(26(31)29-21-9-2-1-3-10-21)24(22)28-17-18-12-13-19-7-4-5-8-20(19)15-18/h1-5,7-10,12-13,15,17H,6,11,14H2,(H,29,31)/b28-17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592448
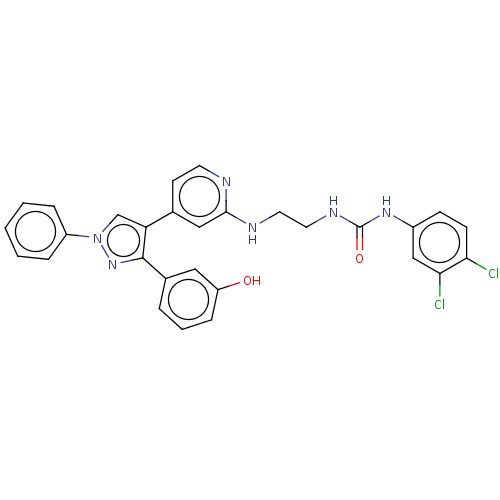
(CHEMBL5183673)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCNC(=O)Nc2ccc(Cl)c(Cl)c2)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 525 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592448

(CHEMBL5183673)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCNC(=O)Nc2ccc(Cl)c(Cl)c2)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50207435

(CHEMBL3960755)Show InChI InChI=1S/C19H18N2O4/c1-24-15-7-3-13(4-8-15)18-19(21(12-20-18)11-17(22)23)14-5-9-16(25-2)10-6-14/h3-10,12H,11H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... |
Bioorg Med Chem 25: 665-676 (2017)
Article DOI: 10.1016/j.bmc.2016.11.037
BindingDB Entry DOI: 10.7270/Q2542QKM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261637

(CHEMBL4092847)Show SMILES O=C(Nc1ccccc1)c1c(N=Cc2cccs2)c(C#N)c2CCCn12 Show InChI InChI=1S/C20H16N4OS/c21-12-16-17-9-4-10-24(17)19(18(16)22-13-15-8-5-11-26-15)20(25)23-14-6-2-1-3-7-14/h1-3,5-8,11,13H,4,9-10H2,(H,23,25)/b22-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592455
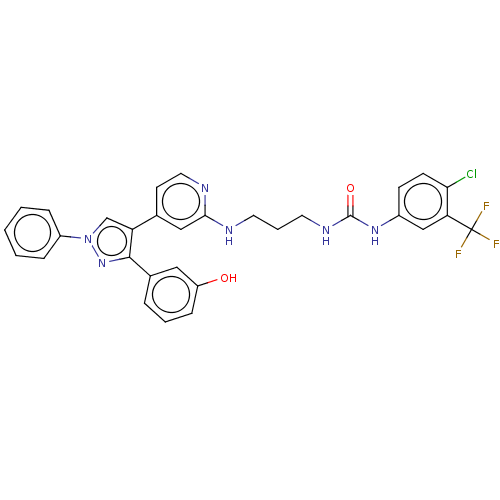
(CHEMBL5179126)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCCNC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 614 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 10
(Homo sapiens (Human)) | BDBM50592455

(CHEMBL5179126)Show SMILES Oc1cccc(c1)-c1nn(cc1-c1ccnc(NCCCNC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 617 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116894
BindingDB Entry DOI: 10.7270/Q2474FVC |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50207430
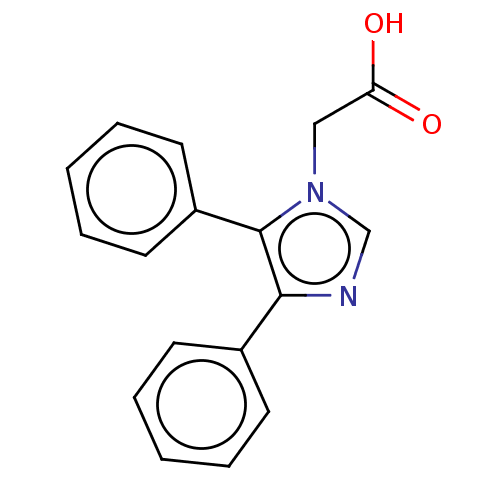
(CHEMBL3924357)Show InChI InChI=1S/C17H14N2O2/c20-15(21)11-19-12-18-16(13-7-3-1-4-8-13)17(19)14-9-5-2-6-10-14/h1-10,12H,11H2,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... |
Bioorg Med Chem 25: 665-676 (2017)
Article DOI: 10.1016/j.bmc.2016.11.037
BindingDB Entry DOI: 10.7270/Q2542QKM |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50261641
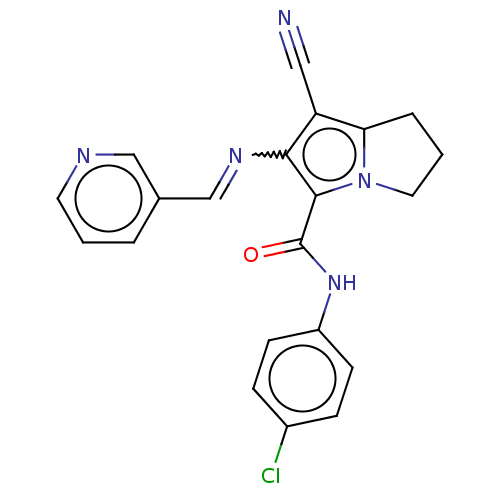
(CHEMBL4074351)Show SMILES Clc1ccc(NC(=O)c2c(N=Cc3cccnc3)c(C#N)c3CCCn23)cc1 Show InChI InChI=1S/C21H16ClN5O/c22-15-5-7-16(8-6-15)26-21(28)20-19(25-13-14-3-1-9-24-12-14)17(11-23)18-4-2-10-27(18)20/h1,3,5-9,12-13H,2,4,10H2,(H,26,28)/b25-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Umm Al-Qura University, Makkah 21955, Saudi Arabia; Department of Medicinal Chemistry, Faculty of Pharmacy, Beni-Suef University, Beni-Sue
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 assessed as reduction in PGH2 formation by measuring PGF2alpha by colorimetric assay |
Bioorg Med Chem 25: 5637-5651 (2017)
Article DOI: 10.1016/j.bmc.2017.08.039
BindingDB Entry DOI: 10.7270/Q2JQ13GQ |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 3
(Homo sapiens (Human)) | BDBM50504116

(CHEMBL4473374)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Oc1ccc(cc1)-c1sc2cc(OS(=O)(=O)c3ccc(cc3)C(C)(C)C)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C48H51NO8S3/c1-47(2,3)35-14-23-40(24-15-35)59(51,52)56-38-20-12-34(13-21-38)46-44(45(50)33-10-18-37(19-11-33)55-31-30-49-28-8-7-9-29-49)42-27-22-39(32-43(42)58-46)57-60(53,54)41-25-16-36(17-26-41)48(4,5)6/h10-27,32H,7-9,28-31H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sharjah
Curated by ChEMBL
| Assay Description
Inhibition of human NPP3 expressed in COS-7 cell membranes assessed as reduction in p-nitrophenol production using pNP-TMP as substrate preincubated ... |
Eur J Med Chem 181: (2019)
Article DOI: 10.1016/j.ejmech.2019.07.063
BindingDB Entry DOI: 10.7270/Q2B85CD9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Beni-Suef University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as reduction in oxidation of TMPD using arachidonic acid as substrate preincubated for 5 mins followed by substrat... |
Bioorg Med Chem 25: 665-676 (2017)
Article DOI: 10.1016/j.bmc.2016.11.037
BindingDB Entry DOI: 10.7270/Q2542QKM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data