 Found 61 hits with Last Name = 'bokesch' and Initial = 'hr'
Found 61 hits with Last Name = 'bokesch' and Initial = 'hr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32628
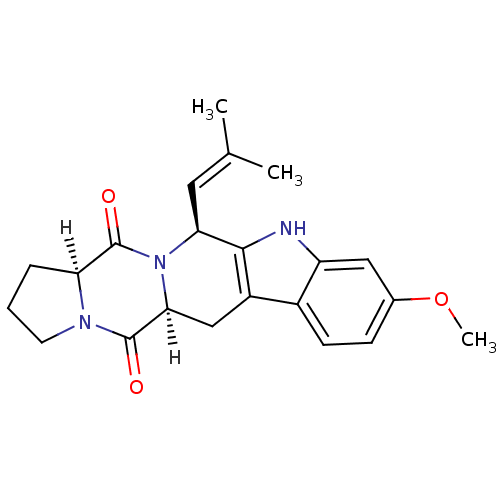
(FTC | Fumitremorgin C)Show SMILES [H][C@@]12CCCN1C(=O)[C@]1([H])Cc3c([nH]c4cc(OC)ccc34)[C@H](C=C(C)C)N1C2=O |wU:8.9,1.0,wD:22.25,(21.14,-14.06,;22.39,-14.97,;22.72,-13.41,;24.31,-13.24,;24.96,-14.7,;23.78,-15.77,;23.76,-17.37,;25.09,-18.16,;22.36,-18.15,;23.69,-18.93,;22.36,-19.69,;21.03,-20.46,;19.7,-19.69,;18.56,-20.72,;19.18,-22.12,;18.51,-23.56,;19.44,-24.86,;18.81,-26.27,;19.7,-27.52,;21.02,-24.7,;21.66,-23.24,;20.71,-21.96,;19.7,-18.15,;18.36,-17.38,;17.03,-18.15,;15.7,-17.38,;17.03,-19.69,;21.03,-17.38,;21,-15.78,;19.66,-15.02,)| Show InChI InChI=1S/C22H25N3O3/c1-12(2)9-18-20-15(14-7-6-13(28-3)10-16(14)23-20)11-19-21(26)24-8-4-5-17(24)22(27)25(18)19/h6-7,9-10,17-19,23H,4-5,8,11H2,1-3H3/t17-,18-,19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32630
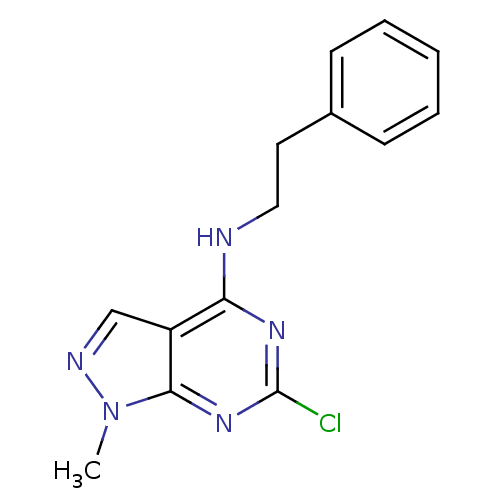
(NSC19139)Show InChI InChI=1S/C14H14ClN5/c1-20-13-11(9-17-20)12(18-14(15)19-13)16-8-7-10-5-3-2-4-6-10/h2-6,9H,7-8H2,1H3,(H,16,18,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32637
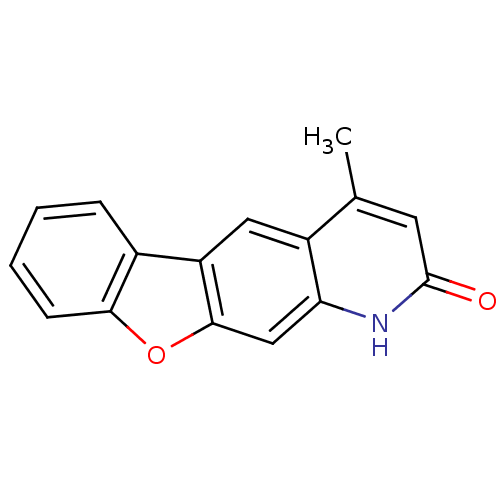
(NSC375985)Show InChI InChI=1S/C16H11NO2/c1-9-6-16(18)17-13-8-15-12(7-11(9)13)10-4-2-3-5-14(10)19-15/h2-8H,1H3,(H,17,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32633

(NSC168201)Show SMILES COc1cc2ccc3c4cc(OC)c(OC)cc4c[n+](C)c3c2cc1OC Show InChI InChI=1S/C22H22NO4/c1-23-12-14-9-19(25-3)20(26-4)10-16(14)15-7-6-13-8-18(24-2)21(27-5)11-17(13)22(15)23/h6-12H,1-5H3/q+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32632
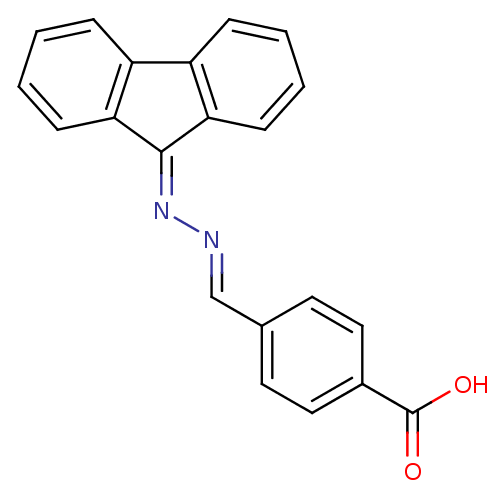
(NSC120688)Show SMILES [#8]-[#6](=O)-c1ccc(\[#6]=[#7]\[#7]=[#6]-2/c3ccccc3-c3ccccc-23)cc1 Show InChI InChI=1S/C21H14N2O2/c24-21(25)15-11-9-14(10-12-15)13-22-23-20-18-7-3-1-5-16(18)17-6-2-4-8-19(17)20/h1-13H,(H,24,25)/b22-13+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32629
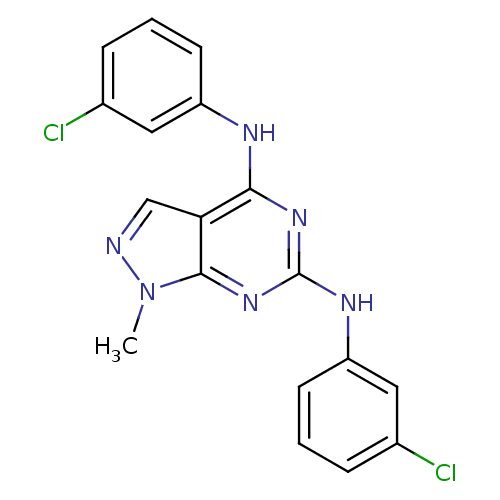
(NSC11668 | cid_223753)Show SMILES Cn1ncc2c(Nc3cccc(Cl)c3)nc(Nc3cccc(Cl)c3)nc12 Show InChI InChI=1S/C18H14Cl2N6/c1-26-17-15(10-21-26)16(22-13-6-2-4-11(19)8-13)24-18(25-17)23-14-7-3-5-12(20)9-14/h2-10H,1H3,(H2,22,23,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32636
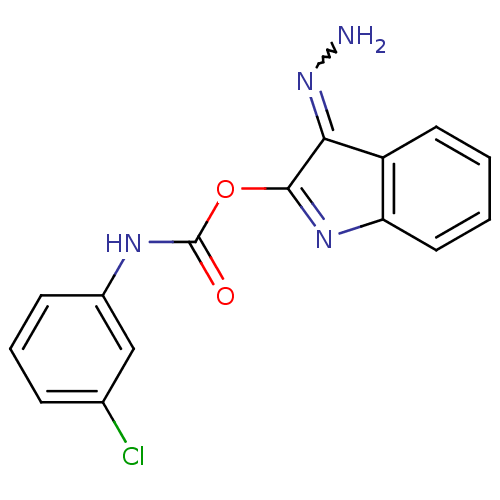
(NSC320852)Show SMILES NN=C1C(OC(=O)Nc2cccc(Cl)c2)=Nc2ccccc12 |w:1.0,c:15| Show InChI InChI=1S/C15H11ClN4O2/c16-9-4-3-5-10(8-9)18-15(21)22-14-13(20-17)11-6-1-2-7-12(11)19-14/h1-8H,17H2,(H,18,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32635
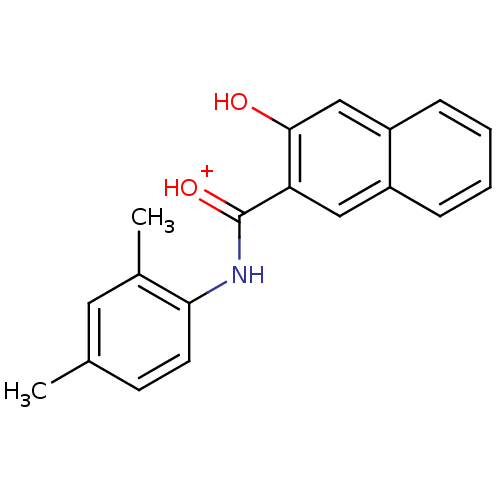
(NSC306698)Show InChI InChI=1S/C19H17NO2/c1-12-7-8-17(13(2)9-12)20-19(22)16-10-14-5-3-4-6-15(14)11-18(16)21/h3-11,21H,1-2H3,(H,20,22)/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32634
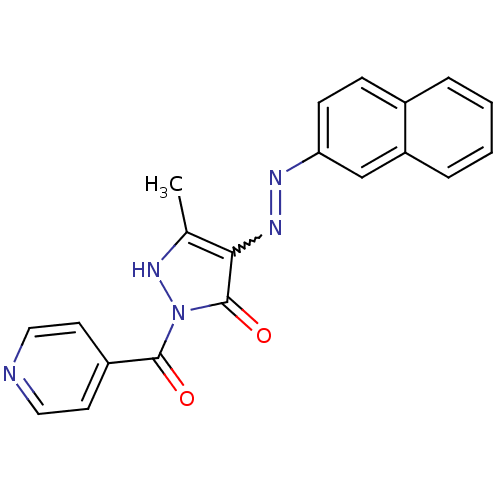
(NSC303769)Show SMILES Cc1[nH]n(C(=O)c2ccncc2)c(=O)c1N=Nc1ccc2ccccc2c1 |w:15.16| Show InChI InChI=1S/C20H15N5O2/c1-13-18(20(27)25(24-13)19(26)15-8-10-21-11-9-15)23-22-17-7-6-14-4-2-3-5-16(14)12-17/h2-12,24H,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50321556

(8-hydroxy-5,10-dimethoxy-2-propyl-4H-benzo[h]chrom...)Show InChI InChI=1S/C18H18O5/c1-4-5-12-9-13(20)17-14(21-2)7-10-6-11(19)8-15(22-3)16(10)18(17)23-12/h6-9,19H,4-5H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2-mediated drug efflux expressed in human NCI-H460 cells |
Bioorg Med Chem Lett 20: 3848-50 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.057
BindingDB Entry DOI: 10.7270/Q2X63NX5 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase CBL-B
(Homo sapiens) | BDBM50581864

(CHEMBL5076820)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]-[#6](=[#6])-[#6](-[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6](=O)-[#7]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#7])-[#8]-[#6](-[#6])=O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent auto-ubiquitination in presence of ATP measured after 13 hrs by fluore... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00367
BindingDB Entry DOI: 10.7270/Q2G73JKR |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase CBL-B
(Homo sapiens) | BDBM50581863

(CHEMBL5093634)Show SMILES CC(=O)OC(CC\C(C)=C\CC\C(C)=C\CC\C(C)=C\C(=O)NCCC[N+](C)(C)CCCC[N+](C)(C)CCCN)C(C)=C | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent auto-ubiquitination in presence of ATP measured after 13 hrs by fluore... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00367
BindingDB Entry DOI: 10.7270/Q2G73JKR |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase CBL-B
(Homo sapiens) | BDBM50581858

(CHEMBL5087523)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6](=O)-[#7]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#7] | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent auto-ubiquitination in presence of ATP measured after 13 hrs by fluore... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00367
BindingDB Entry DOI: 10.7270/Q2G73JKR |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase CBL-B
(Homo sapiens) | BDBM50581859

(CHEMBL5083569)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6] | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent auto-ubiquitination in presence of ATP measured after 13 hrs by fluore... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00367
BindingDB Entry DOI: 10.7270/Q2G73JKR |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase CBL-B
(Homo sapiens) | BDBM50581862
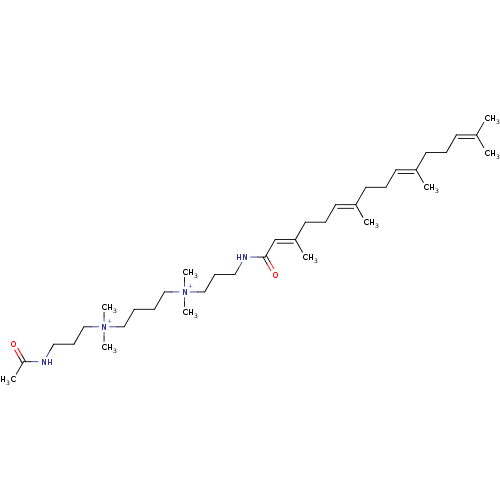
(CHEMBL5092374)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6](=O)-[#7]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#7]-[#6](-[#6])=O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent auto-ubiquitination in presence of ATP measured after 13 hrs by fluore... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00367
BindingDB Entry DOI: 10.7270/Q2G73JKR |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase CBL-B
(Homo sapiens) | BDBM50581861

(CHEMBL5089524)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6](=O)-[#7]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#7]-[#6]=O | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent auto-ubiquitination in presence of ATP measured after 13 hrs by fluore... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00367
BindingDB Entry DOI: 10.7270/Q2G73JKR |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase CBL-B
(Homo sapiens) | BDBM50581860

(CHEMBL5078551)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6](=O)-[#7]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#6][N+]([#6])([#6])[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6] | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Cbl-b (unknown origin) assessed as inhibition of Cbl-b dependent auto-ubiquitination in presence of ATP measured after 13 hrs by fluore... |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c00367
BindingDB Entry DOI: 10.7270/Q2G73JKR |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32624

(Botryllamide G, 7 | US8470888, Botryllamide G)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1cc(Br)c(O)c(Br)c1 Show InChI InChI=1S/C18H15Br2NO4/c1-25-16(10-11-2-4-13(22)5-3-11)18(24)21-7-6-12-8-14(19)17(23)15(20)9-12/h2-10,22-23H,1H3,(H,21,24)/b7-6+,16-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32624

(Botryllamide G, 7 | US8470888, Botryllamide G)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1cc(Br)c(O)c(Br)c1 Show InChI InChI=1S/C18H15Br2NO4/c1-25-16(10-11-2-4-13(22)5-3-11)18(24)21-7-6-12-8-14(19)17(23)15(20)9-12/h2-10,22-23H,1H3,(H,21,24)/b7-6+,16-10- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services
US Patent
| Assay Description
Inhibition assay using ABCG2 or BCRP1. |
US Patent US8470888 (2013)
BindingDB Entry DOI: 10.7270/Q2RR1WWT |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50221718
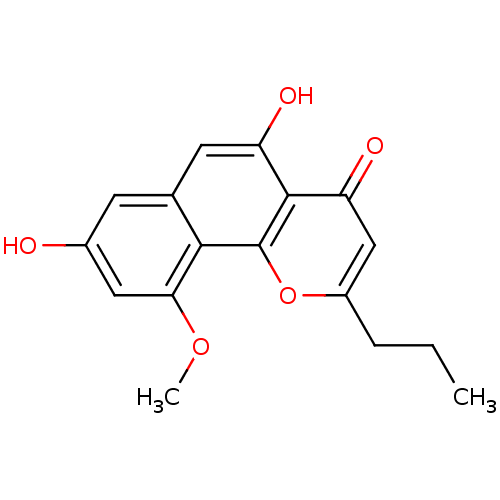
(CHEMBL400538 | comaparvin)Show InChI InChI=1S/C17H16O5/c1-3-4-11-8-13(20)16-12(19)6-9-5-10(18)7-14(21-2)15(9)17(16)22-11/h5-8,18-19H,3-4H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2-mediated drug efflux expressed in human NCI-H460 cells |
Bioorg Med Chem Lett 20: 3848-50 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.057
BindingDB Entry DOI: 10.7270/Q2X63NX5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32631
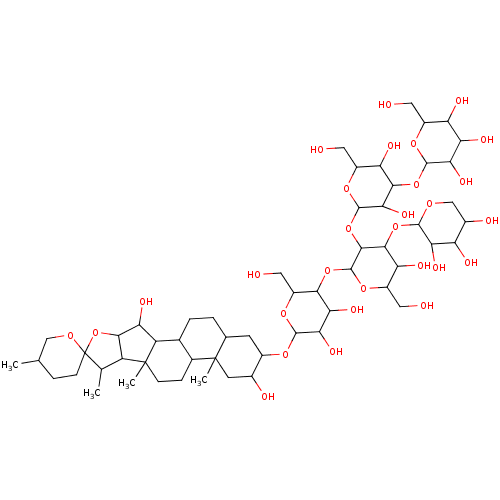
(Digitonin | NSC23471)Show SMILES CC1C2C(OC11CCC(C)CO1)C(O)C1C3CCC4CC(OC5OC(CO)C(OC6OC(CO)C(O)C(OC7OCC(O)C(O)C7O)C6OC6OC(CO)C(O)C(OC7OC(CO)C(O)C(O)C7O)C6O)C(O)C5O)C(O)CC4(C)C3CCC21C Show InChI InChI=1S/C56H92O29/c1-19-7-10-56(75-17-19)20(2)31-45(85-56)37(67)32-22-6-5-21-11-26(24(61)12-55(21,4)23(22)8-9-54(31,32)3)76-50-42(72)39(69)44(30(16-60)80-50)81-53-48(47(36(66)29(15-59)79-53)83-49-40(70)33(63)25(62)18-74-49)84-52-43(73)46(35(65)28(14-58)78-52)82-51-41(71)38(68)34(64)27(13-57)77-51/h19-53,57-73H,5-18H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32619

(Botryllamide B, 2)Show SMILES CO\C(=C\c1ccc(O)cc1)C(=O)N\C=C\c1cc(Br)c(OC)c(Br)c1 Show InChI InChI=1S/C19H17Br2NO4/c1-25-17(11-12-3-5-14(23)6-4-12)19(24)22-8-7-13-9-15(20)18(26-2)16(21)10-13/h3-11,23H,1-2H3,(H,22,24)/b8-7+,17-11+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32617

(Botryllamide A, 1 | US8470888, Botryllamide A)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1cc(Br)c(OC)c(Br)c1 Show InChI InChI=1S/C19H17Br2NO4/c1-25-17(11-12-3-5-14(23)6-4-12)19(24)22-8-7-13-9-15(20)18(26-2)16(21)10-13/h3-11,23H,1-2H3,(H,22,24)/b8-7+,17-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services
US Patent
| Assay Description
Inhibition assay using ABCG2 or BCRP1. |
US Patent US8470888 (2013)
BindingDB Entry DOI: 10.7270/Q2RR1WWT |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32638

(Dihydroergocristine | NSC409663)Show SMILES CC(C)C1(NC(=O)C2CC3C(Cc4c[nH]c5cccc3c45)N(C)C2)OC2(O)C3CCCN3C(=O)C(Cc3ccccc3)N2C1=O Show InChI InChI=1S/C35H41N5O5/c1-20(2)34(37-31(41)23-16-25-24-11-7-12-26-30(24)22(18-36-26)17-27(25)38(3)19-23)33(43)40-28(15-21-9-5-4-6-10-21)32(42)39-14-8-13-29(39)35(40,44)45-34/h4-7,9-12,18,20,23,25,27-29,36,44H,8,13-17,19H2,1-3H3,(H,37,41) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
Mol Cancer Ther 6: 3271-8 (2007)
Article DOI: 10.1158/1535-7163.MCT-07-0352
BindingDB Entry DOI: 10.7270/Q21C1V7K |
More data for this
Ligand-Target Pair | |
Small ubiquitin-related modifier 1
(Homo sapiens (Human)) | BDBM50366295

(CHEMBL4163902)Show SMILES C[S+]([O-])C1=C(O)C(=O)c2[nH]cc(CO)c2C1=N |c:3| Show InChI InChI=1S/C10H10N2O4S/c1-17(16)10-6(11)5-4(3-13)2-12-7(5)8(14)9(10)15/h2,11-13,15H,3H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged SUMO1 expressed in Escherichia coli assessed as reduction in FL-AR peptide sumoylation measure... |
J Nat Prod 81: 1666-1672 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00343
BindingDB Entry DOI: 10.7270/Q2028V31 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50321557
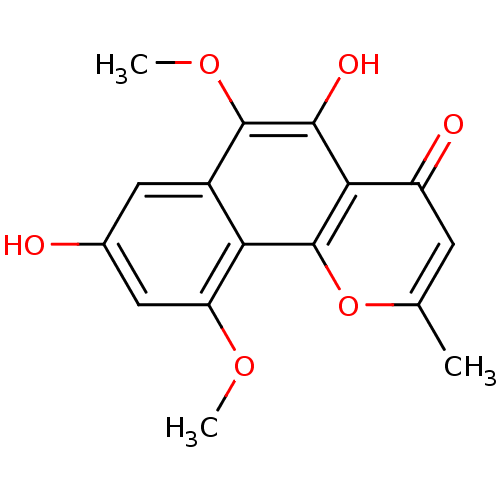
(5,8-dihydroxy-6,10-dimethoxy-2-methyl-4H-benzo[h]c...)Show InChI InChI=1S/C16H14O6/c1-7-4-10(18)13-14(19)15(21-3)9-5-8(17)6-11(20-2)12(9)16(13)22-7/h4-6,17,19H,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2-mediated drug efflux expressed in human NCI-H460 cells |
Bioorg Med Chem Lett 20: 3848-50 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.057
BindingDB Entry DOI: 10.7270/Q2X63NX5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50242177
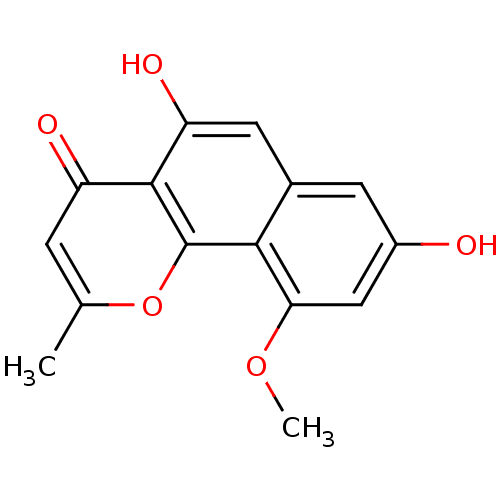
(5,8-dihydroxy-10-methoxy-2-methyl-4H-benzo[h]chrom...)Show InChI InChI=1S/C15H12O5/c1-7-3-10(17)14-11(18)5-8-4-9(16)6-12(19-2)13(8)15(14)20-7/h3-6,16,18H,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2-mediated drug efflux expressed in human NCI-H460 cells |
Bioorg Med Chem Lett 20: 3848-50 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.057
BindingDB Entry DOI: 10.7270/Q2X63NX5 |
More data for this
Ligand-Target Pair | |
PAX33/FOXO11
(Homo sapiens (Human)) | BDBM50595105

(CHEMBL5192045)Show SMILES CC1c2[nH]c(NC(=O)c3ccccc3)nc2CCc2nc(NC(=O)c3ccccc3)[nH]c12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00246
BindingDB Entry DOI: 10.7270/Q2ZG6X85 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32620
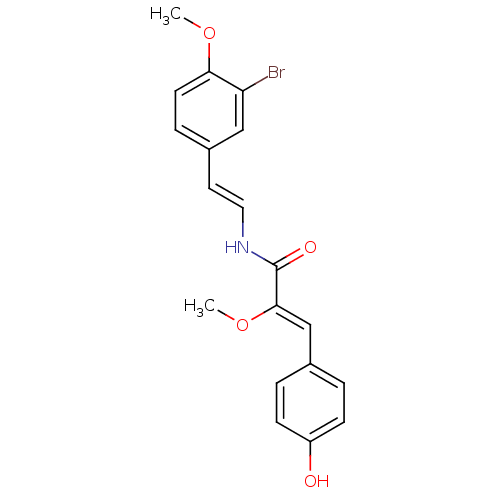
(Botryllamide C, 3 | US8470888, Botryllamide D)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1ccc(OC)c(Br)c1 Show InChI InChI=1S/C19H18BrNO4/c1-24-17-8-5-14(11-16(17)20)9-10-21-19(23)18(25-2)12-13-3-6-15(22)7-4-13/h3-12,22H,1-2H3,(H,21,23)/b10-9+,18-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services
US Patent
| Assay Description
Inhibition assay using ABCG2 or BCRP1. |
US Patent US8470888 (2013)
BindingDB Entry DOI: 10.7270/Q2RR1WWT |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32621
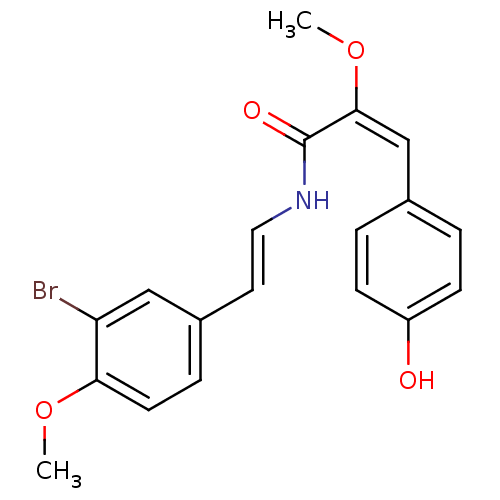
(Botryllamide D, 4)Show SMILES CO\C(=C\c1ccc(O)cc1)C(=O)N\C=C\c1ccc(OC)c(Br)c1 Show InChI InChI=1S/C19H18BrNO4/c1-24-17-8-5-14(11-16(17)20)9-10-21-19(23)18(25-2)12-13-3-6-15(22)7-4-13/h3-12,22H,1-2H3,(H,21,23)/b10-9+,18-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50321555
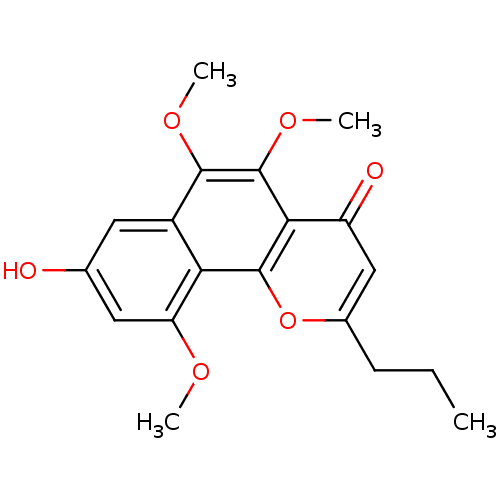
(6-methoxycomaparvin 5-methyl ether | CHEMBL256967)Show InChI InChI=1S/C19H20O6/c1-5-6-11-9-13(21)16-18(25-11)15-12(17(23-3)19(16)24-4)7-10(20)8-14(15)22-2/h7-9,20H,5-6H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2-mediated drug efflux expressed in human NCI-H460 cells |
Bioorg Med Chem Lett 20: 3848-50 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.057
BindingDB Entry DOI: 10.7270/Q2X63NX5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32623
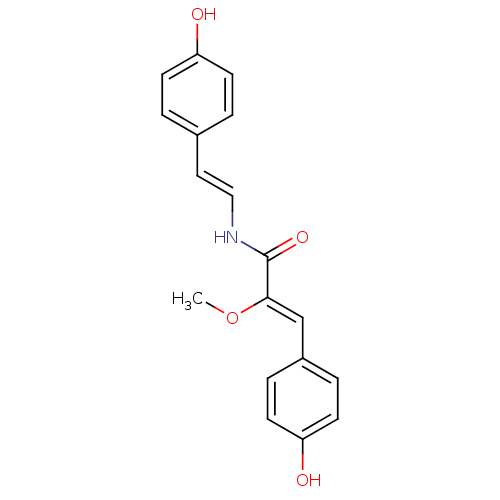
(Botryllamide F, 6 | US8470888, Botryllamide F)Show InChI InChI=1S/C18H17NO4/c1-23-17(12-14-4-8-16(21)9-5-14)18(22)19-11-10-13-2-6-15(20)7-3-13/h2-12,20-21H,1H3,(H,19,22)/b11-10+,17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services
US Patent
| Assay Description
Inhibition assay using ABCG2 or BCRP1. |
US Patent US8470888 (2013)
BindingDB Entry DOI: 10.7270/Q2RR1WWT |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32623

(Botryllamide F, 6 | US8470888, Botryllamide F)Show InChI InChI=1S/C18H17NO4/c1-23-17(12-14-4-8-16(21)9-5-14)18(22)19-11-10-13-2-6-15(20)7-3-13/h2-12,20-21H,1H3,(H,19,22)/b11-10+,17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Paired box protein Pax-3
(Homo sapiens) | BDBM50595107

(CHEMBL5185415)Show SMILES CC1c2[nH]c(NC(=O)C=C(C)C)nc2CCc2nc(NC(=O)c3ccccc3)[nH]c12 |(-.14,2.58,;-.14,1.04,;-1.55,.43,;-2.85,1.26,;-4.07,.29,;-5.56,.69,;-6.65,-.4,;-6.25,-1.89,;-8.14,-0,;-9.23,-1.09,;-10.72,-.69,;-8.83,-2.58,;-3.54,-1.13,;-1.95,-1.07,;-1.03,-2.37,;.51,-2.37,;1.51,-1.19,;3.02,-1.38,;3.68,0,;5.17,.4,;6.26,-.69,;5.86,-2.18,;7.74,-.29,;8.14,1.2,;9.63,1.59,;10.72,.5,;10.32,-.98,;8.84,-1.38,;2.58,1.05,;1.22,.32,)| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00246
BindingDB Entry DOI: 10.7270/Q2ZG6X85 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM50321558
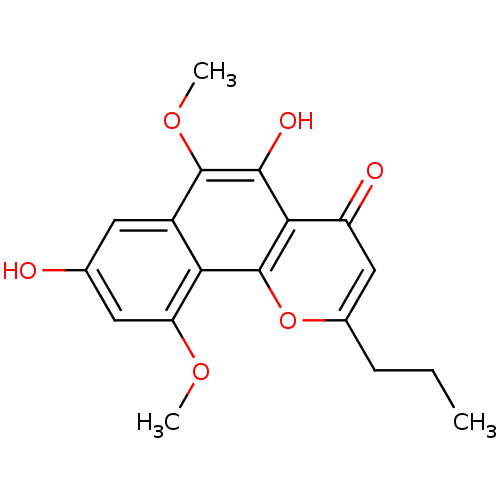
(6-methoxycomaparvin | CHEMBL401565)Show InChI InChI=1S/C18H18O6/c1-4-5-10-8-12(20)15-16(21)17(23-3)11-6-9(19)7-13(22-2)14(11)18(15)24-10/h6-8,19,21H,4-5H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NCI-Frederick
Curated by ChEMBL
| Assay Description
Inhibition of ABCG2-mediated drug efflux expressed in human NCI-H460 cells |
Bioorg Med Chem Lett 20: 3848-50 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.057
BindingDB Entry DOI: 10.7270/Q2X63NX5 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32622
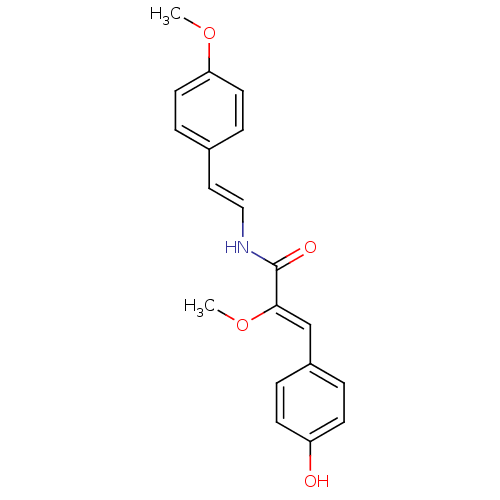
(Botryllamide E, 5 | US8470888, Botryllamide I)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1ccc(OC)cc1 Show InChI InChI=1S/C19H19NO4/c1-23-17-9-5-14(6-10-17)11-12-20-19(22)18(24-2)13-15-3-7-16(21)8-4-15/h3-13,21H,1-2H3,(H,20,22)/b12-11+,18-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services
US Patent
| Assay Description
Inhibition assay using ABCG2 or BCRP1. |
US Patent US8470888 (2013)
BindingDB Entry DOI: 10.7270/Q2RR1WWT |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32622

(Botryllamide E, 5 | US8470888, Botryllamide I)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1ccc(OC)cc1 Show InChI InChI=1S/C19H19NO4/c1-23-17-9-5-14(6-10-17)11-12-20-19(22)18(24-2)13-15-3-7-16(21)8-4-15/h3-13,21H,1-2H3,(H,20,22)/b12-11+,18-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Paired box protein Pax-3
(Homo sapiens) | BDBM50595109
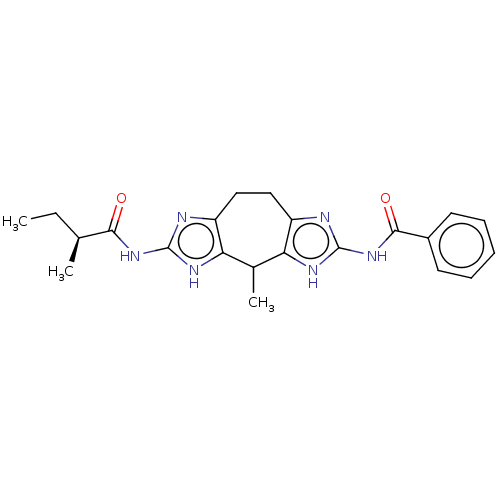
(CHEMBL5185993)Show SMILES CC[C@H](C)C(=O)Nc1nc2CCc3nc(NC(=O)c4ccccc4)[nH]c3C(C)c2[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00246
BindingDB Entry DOI: 10.7270/Q2ZG6X85 |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32625

(Botryllamide H, 8 | Botryllamide J, 10)Show SMILES Oc1ccc(C=CNC(=O)C(=O)Cc2cccc(O)c2C#N)cc1 |w:5.4| Show InChI InChI=1S/C18H14N2O4/c19-11-15-13(2-1-3-16(15)22)10-17(23)18(24)20-9-8-12-4-6-14(21)7-5-12/h1-9,21-22H,10H2,(H,20,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM97639
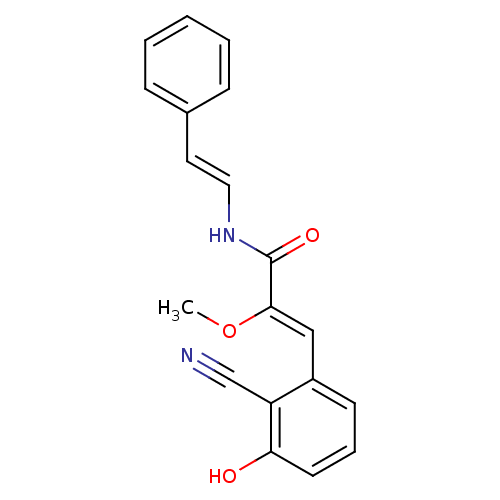
(US8470888, Botryllamide J)Show InChI InChI=1S/C19H16N2O3/c1-24-18(12-15-8-5-9-17(22)16(15)13-20)19(23)21-11-10-14-6-3-2-4-7-14/h2-12,22H,1H3,(H,21,23)/b11-10+,18-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services
US Patent
| Assay Description
Inhibition assay using ABCG2 or BCRP1. |
US Patent US8470888 (2013)
BindingDB Entry DOI: 10.7270/Q2RR1WWT |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32617

(Botryllamide A, 1 | US8470888, Botryllamide A)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1cc(Br)c(OC)c(Br)c1 Show InChI InChI=1S/C19H17Br2NO4/c1-25-17(11-12-3-5-14(23)6-4-12)19(24)22-8-7-13-9-15(20)18(26-2)16(21)10-13/h3-11,23H,1-2H3,(H,22,24)/b8-7+,17-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services
US Patent
| Assay Description
Inhibition assay using ABCG2 or BCRP1. |
US Patent US8470888 (2013)
BindingDB Entry DOI: 10.7270/Q2RR1WWT |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32617

(Botryllamide A, 1 | US8470888, Botryllamide A)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1cc(Br)c(OC)c(Br)c1 Show InChI InChI=1S/C19H17Br2NO4/c1-25-17(11-12-3-5-14(23)6-4-12)19(24)22-8-7-13-9-15(20)18(26-2)16(21)10-13/h3-11,23H,1-2H3,(H,22,24)/b8-7+,17-11- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.34E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32626
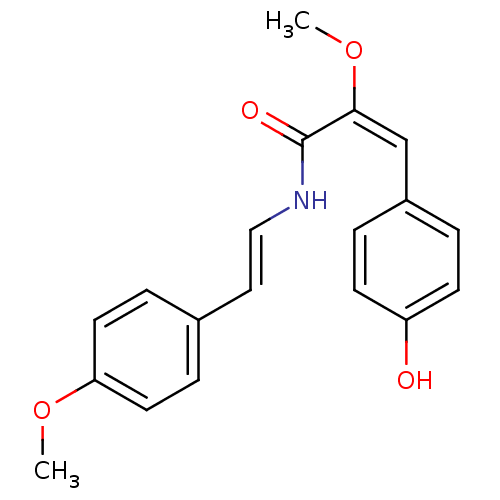
(Botryllamide I, 9)Show SMILES CO\C(=C\c1ccc(O)cc1)C(=O)N\C=C\c1ccc(OC)cc1 Show InChI InChI=1S/C19H19NO4/c1-23-17-9-5-14(6-10-17)11-12-20-19(22)18(24-2)13-15-3-7-16(21)8-4-15/h3-13,21H,1-2H3,(H,20,22)/b12-11+,18-13+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.14E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32622

(Botryllamide E, 5 | US8470888, Botryllamide I)Show SMILES CO\C(=C/c1ccc(O)cc1)C(=O)N\C=C\c1ccc(OC)cc1 Show InChI InChI=1S/C19H19NO4/c1-23-17-9-5-14(6-10-17)11-12-20-19(22)18(24-2)13-15-3-7-16(21)8-4-15/h3-13,21H,1-2H3,(H,20,22)/b12-11+,18-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services
US Patent
| Assay Description
Inhibition assay using ABCG2 or BCRP1. |
US Patent US8470888 (2013)
BindingDB Entry DOI: 10.7270/Q2RR1WWT |
More data for this
Ligand-Target Pair | |
Paired box protein Pax-3
(Homo sapiens) | BDBM50595106

(CHEMBL5180582)Show SMILES CC[C@H](C)C(=O)Nc1nc2CCc3nc(NC(=O)\C=C\c4ccccc4)[nH]c3C(C)c2[nH]1 |r| | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00246
BindingDB Entry DOI: 10.7270/Q2ZG6X85 |
More data for this
Ligand-Target Pair | |
Paired box protein Pax-3
(Homo sapiens) | BDBM50595108

(CHEMBL5175239)Show SMILES CC(C)C(=O)Nc1nc2CCc3nc(NC(=O)c4ccccc4)[nH]c3C(C)c2[nH]1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.2c00246
BindingDB Entry DOI: 10.7270/Q2ZG6X85 |
More data for this
Ligand-Target Pair | |
Small ubiquitin-related modifier 1
(Homo sapiens (Human)) | BDBM50366293

(CHEMBL4166477)Show InChI InChI=1S/C12H11N3OS/c16-11-8-7-6(5-15-8)1-2-13-9(7)12-10(11)14-3-4-17-12/h5,14-15H,1-4H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged SUMO1 expressed in Escherichia coli assessed as reduction in FL-AR peptide sumoylation measure... |
J Nat Prod 81: 1666-1672 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00343
BindingDB Entry DOI: 10.7270/Q2028V31 |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1
(Homo sapiens (Human)) | BDBM50398329
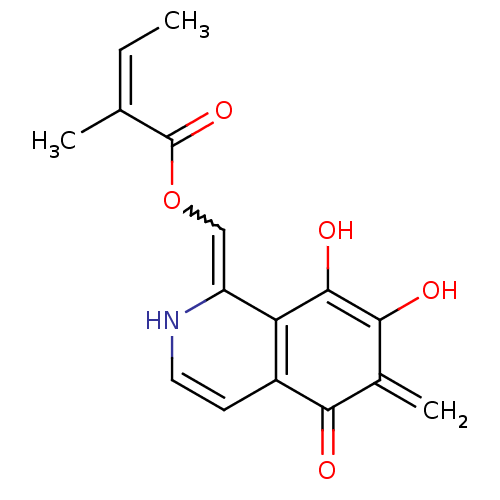
(CHEMBL2177347)Show SMILES C\C=C(\C)C(=O)OC=c1[nH]ccc2c1c(O)c(O)c(=C)c2=O |w:7.6| Show InChI InChI=1S/C16H15NO5/c1-4-8(2)16(21)22-7-11-12-10(5-6-17-11)13(18)9(3)14(19)15(12)20/h4-7,17,19-20H,3H2,1-2H3/b8-4-,11-7? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of HIF2alpha in human 786-0 cells expresseing truncated HIF1alpha assessed as reduction in luciferase activity after 24 hrs by reporter ge... |
J Nat Prod 75: 1632-6 (2012)
Article DOI: 10.1021/np300211x
BindingDB Entry DOI: 10.7270/Q2HM59KQ |
More data for this
Ligand-Target Pair | |
Broad substrate specificity ATP-binding cassette transporter ABCG2
(Homo sapiens (Human)) | BDBM32625

(Botryllamide H, 8 | Botryllamide J, 10)Show SMILES Oc1ccc(C=CNC(=O)C(=O)Cc2cccc(O)c2C#N)cc1 |w:5.4| Show InChI InChI=1S/C18H14N2O4/c19-11-15-13(2-1-3-16(15)22)10-17(23)18(24)20-9-8-12-4-6-14(21)7-5-12/h1-9,21-22H,10H2,(H,20,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
SAIC-Frederick
| Assay Description
IC50 and maximal activities for inhibition of PhA accumulation were determined from dose-response curves. The accumulation of PhA, a fluorescent ABCG... |
ACS Chem Biol 4: 637-47 (2009)
Article DOI: 10.1021/cb900134c
BindingDB Entry DOI: 10.7270/Q2542KZW |
More data for this
Ligand-Target Pair | |
Endothelial PAS domain-containing protein 1
(Homo sapiens (Human)) | BDBM50398328
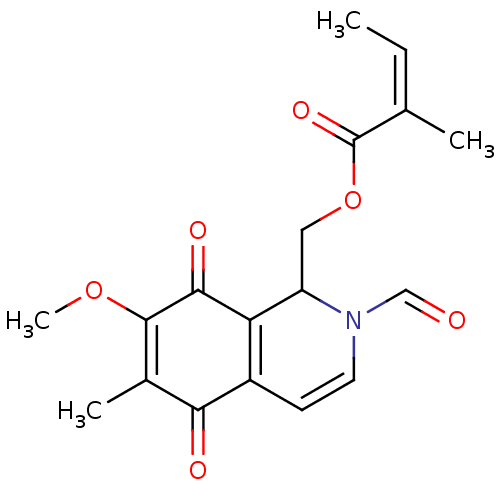
(CHEMBL2177348)Show SMILES COC1=C(C)C(=O)C2=C(C(COC(=O)C(\C)=C/C)N(C=O)C=C2)C1=O |c:2,21,t:7| Show InChI InChI=1S/C18H19NO6/c1-5-10(2)18(23)25-8-13-14-12(6-7-19(13)9-20)15(21)11(3)17(24-4)16(14)22/h5-7,9,13H,8H2,1-4H3/b10-5- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
Frederick National Laboratory for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of HIF2alpha in human 786-0 cells expresseing truncated HIF1alpha assessed as reduction in luciferase activity after 24 hrs by reporter ge... |
J Nat Prod 75: 1632-6 (2012)
Article DOI: 10.1021/np300211x
BindingDB Entry DOI: 10.7270/Q2HM59KQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data