

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020515 (2-(Hydroxyimino-methyl)-3-methyl-1-(naphthalen-1-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of phosphorylation of Eel acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020529 (2-(Hydroxyimino-methyl)-3-methyl-1-(3-phenyl-propo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of phosphonylation of Eel acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020521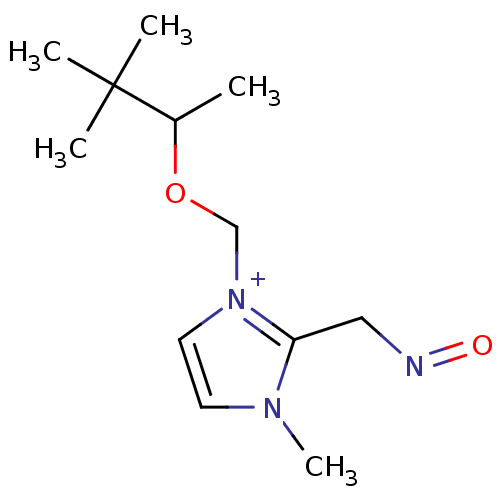 (2-(Hydroxyimino-methyl)-3-methyl-1-(1,2,2-trimethy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of phosphonylation of Eel acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020514 (2-(Hydroxyimino-methyl)-1-methoxymethyl-3-methyl-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of phosphorylation of Eel acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of phosphorylation of Eel acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020522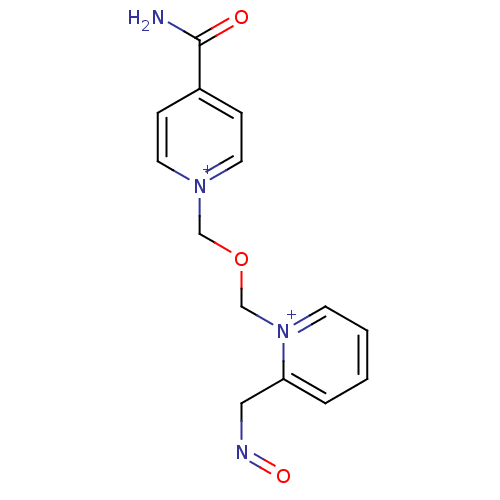 (1-(((4-carbamoylpyridinium-1-yl)methoxy)methyl)-2-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of phosphonylation of Eel acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025238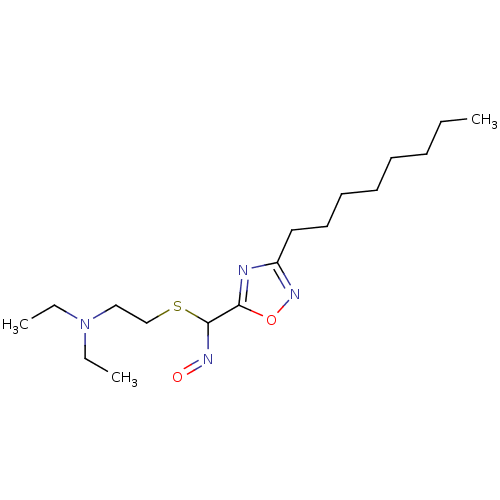 (CHEMBL71740 | N-Hydroxy-3-octyl-[1,2,4]oxadiazole-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the concentration required for reversible inhibition of human acetylcholinesterase . | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025251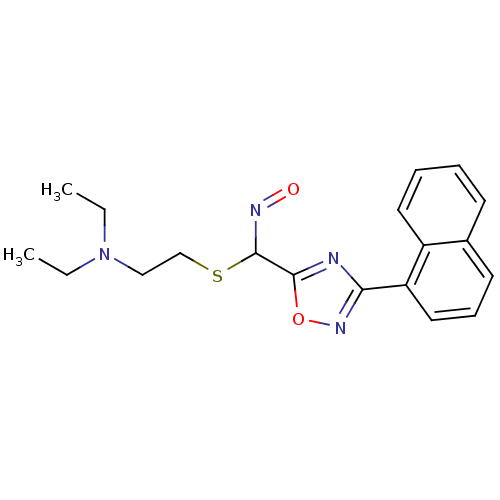 (CHEMBL302946 | N-Hydroxy-3-naphthalen-1-yl-[1,2,4]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025238 (CHEMBL71740 | N-Hydroxy-3-octyl-[1,2,4]oxadiazole-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for the concentration required for reversible inhibition of eel acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020516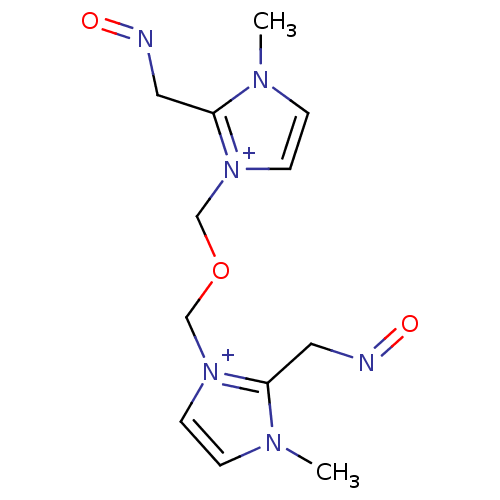 (2-(3-{2-[2-hydroxy-(E)-1-methenylimino]-1-methyl-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020515 (2-(Hydroxyimino-methyl)-3-methyl-1-(naphthalen-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50020515 (2-(Hydroxyimino-methyl)-3-methyl-1-(naphthalen-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in bovine erythrocyte(RBC) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025242 (CHEMBL20339 | CHEMBL2079594 | N-Hydroxy-3-phenyl-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Reactivation of human acetylcholinesterase | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020515 (2-(Hydroxyimino-methyl)-3-methyl-1-(naphthalen-1-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020517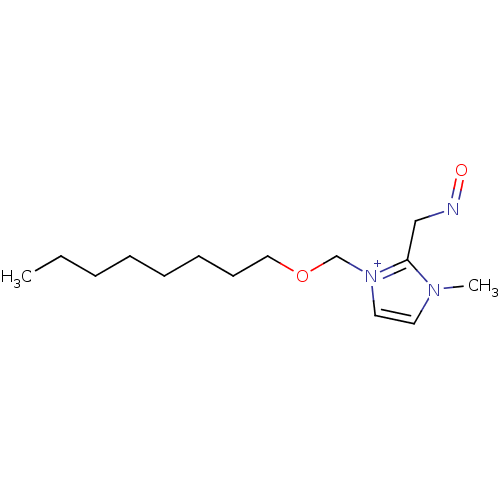 (2-(Hydroxyimino-methyl)-3-methyl-1-octyloxymethyl-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50020515 (2-(Hydroxyimino-methyl)-3-methyl-1-(naphthalen-1-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025242 (CHEMBL20339 | CHEMBL2079594 | N-Hydroxy-3-phenyl-[...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020516 (2-(3-{2-[2-hydroxy-(E)-1-methenylimino]-1-methyl-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020519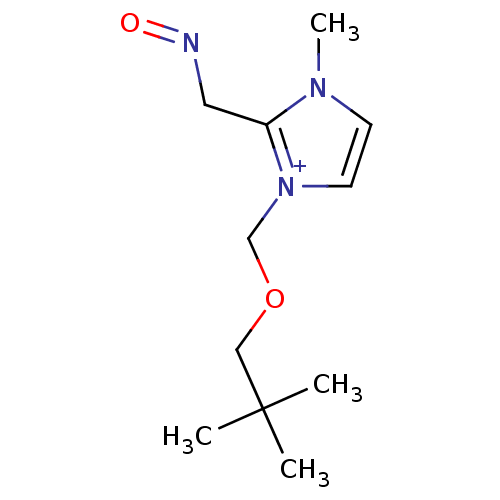 (1-(2,2-Dimethyl-propoxymethyl)-2-(hydroxyimino-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50020519 (1-(2,2-Dimethyl-propoxymethyl)-2-(hydroxyimino-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in bovine erythrocyte(RBC) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020517 (2-(Hydroxyimino-methyl)-3-methyl-1-octyloxymethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50020517 (2-(Hydroxyimino-methyl)-3-methyl-1-octyloxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in bovine erythrocyte(RBC) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020521 (2-(Hydroxyimino-methyl)-3-methyl-1-(1,2,2-trimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50367852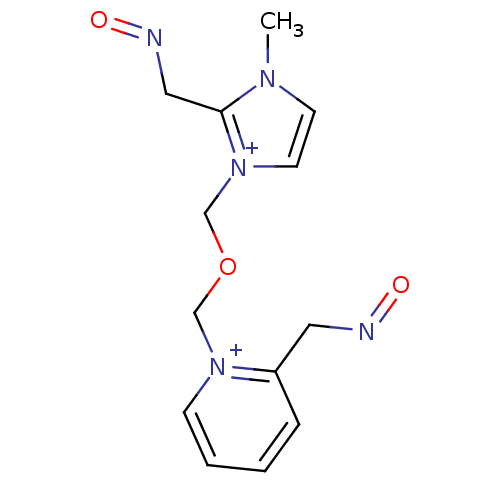 (CHEMBL538352) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020519 (1-(2,2-Dimethyl-propoxymethyl)-2-(hydroxyimino-met...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50020529 (2-(Hydroxyimino-methyl)-3-methyl-1-(3-phenyl-propo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020529 (2-(Hydroxyimino-methyl)-3-methyl-1-(3-phenyl-propo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020521 (2-(Hydroxyimino-methyl)-3-methyl-1-(1,2,2-trimethy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of phosphonylation of Eel acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Choline O-acetyltransferase (RAT) | BDBM50020521 (2-(Hydroxyimino-methyl)-3-methyl-1-(1,2,2-trimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the competitive inhibition of acetylcholinesterase (AChE) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50020521 (2-(Hydroxyimino-methyl)-3-methyl-1-(1,2,2-trimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in bovine erythrocyte(RBC) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020518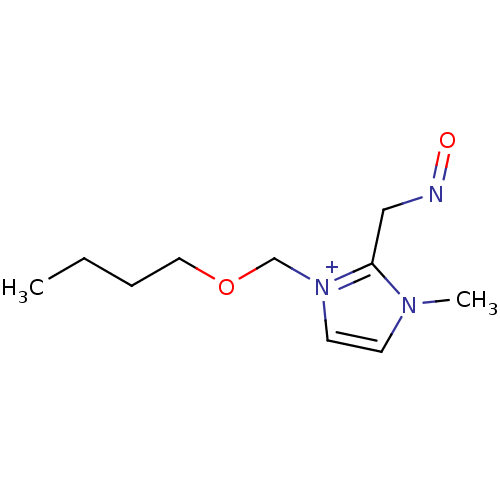 (1-Butoxymethyl-2-(hydroxyimino-methyl)-3-methyl-3H...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020529 (2-(Hydroxyimino-methyl)-3-methyl-1-(3-phenyl-propo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (Mus musculus-MOUSE) | BDBM50020529 (2-(Hydroxyimino-methyl)-3-methyl-1-(3-phenyl-propo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the binding affinity towards muscarinic receptor in mouse brain membrane | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020534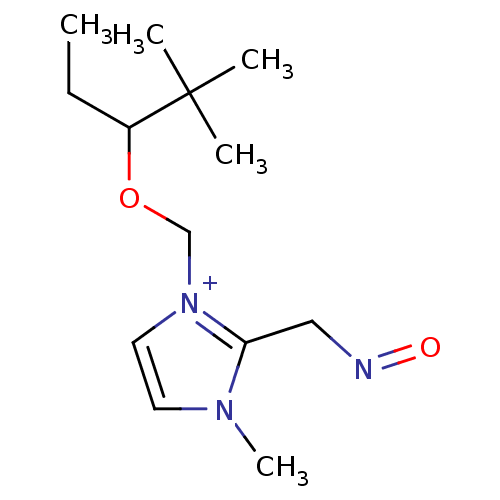 (1-(1-Ethyl-2,2-dimethyl-propoxymethyl)-2-(hydroxyi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020535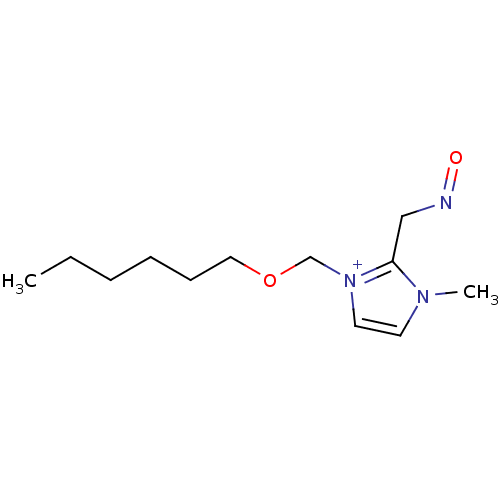 (1-Hexyloxymethyl-2-(hydroxyimino-methyl)-3-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020533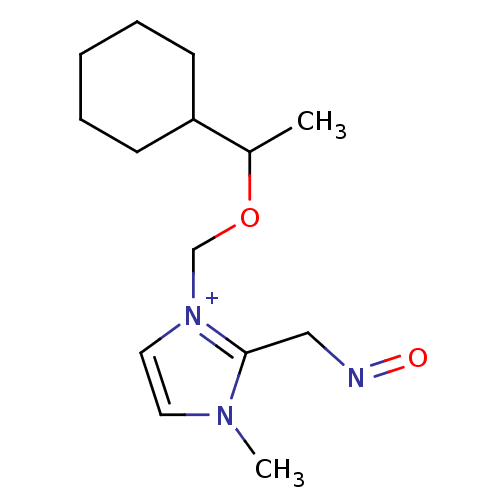 (1-(1-Cyclohexyl-ethoxymethyl)-2-(hydroxyimino-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020518 (1-Butoxymethyl-2-(hydroxyimino-methyl)-3-methyl-3H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50020529 (2-(Hydroxyimino-methyl)-3-methyl-1-(3-phenyl-propo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in bovine erythrocyte(RBC) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020530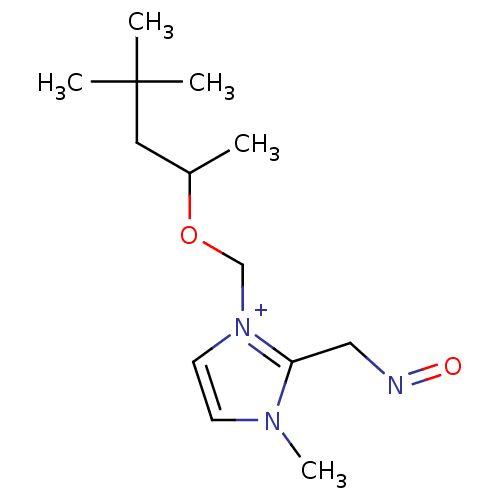 (2-(Hydroxyimino-methyl)-3-methyl-1-(1,3,3-trimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020531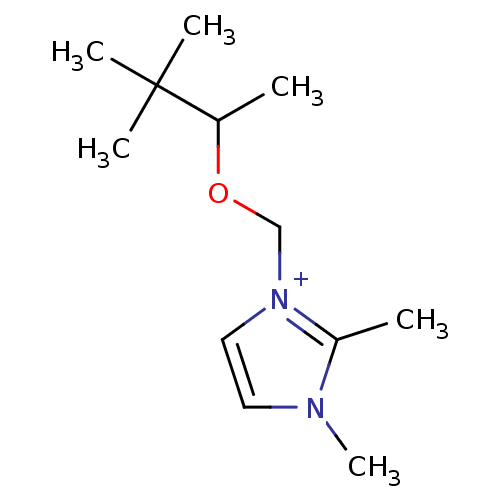 (2,3-Dimethyl-1-(1,2,2-trimethyl-propoxymethyl)-3H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (Mus musculus-MOUSE) | BDBM50020515 (2-(Hydroxyimino-methyl)-3-methyl-1-(naphthalen-1-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Compound was tested for the binding affinity towards muscarinic receptor in mouse brain membrane | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50367852 (CHEMBL538352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020528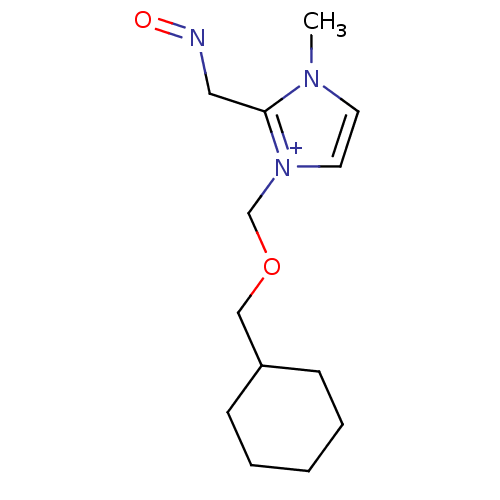 (1-Cyclohexylmethoxymethyl-2-(hydroxyimino-methyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50020518 (1-Butoxymethyl-2-(hydroxyimino-methyl)-3-methyl-3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in bovine erythrocyte(RBC) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020527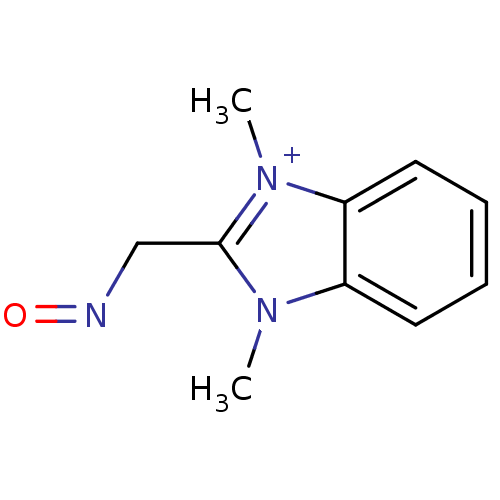 (2-(Hydroxyimino-methyl)-1,3-dimethyl-3H-benzoimida...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50020520 (2-(Hydroxyimino-methyl)-1-isopropoxymethyl-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (AChE) in bovine erythrocyte(RBC) | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50020520 (2-(Hydroxyimino-methyl)-1-isopropoxymethyl-3-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration required to inhibit 50% of acetylcholinesterase (AChE) in human erythrocyte(RBC). | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50020523 (1-Benzyloxymethyl-2-(hydroxyimino-methyl)-3-methyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of eel acetylcholinesterase (AChE) activity by 50% | J Med Chem 32: 493-503 (1989) BindingDB Entry DOI: 10.7270/Q2JD4XCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50025239 (3-Benzyl-N-hydroxy-[1,2,4]oxadiazole-5-carboximido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50025239 (3-Benzyl-N-hydroxy-[1,2,4]oxadiazole-5-carboximido...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro reversible inhibition of eel acetylcholinesterase. | J Med Chem 29: 2174-83 (1986) BindingDB Entry DOI: 10.7270/Q2VQ31QK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 127 total ) | Next | Last >> |