

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313166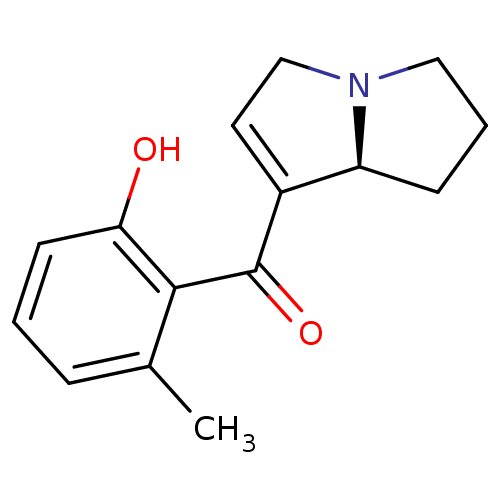 ((S)-(2-hydroxy-6-methylphenyl)(2,3,5,7a-tetrahydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50313167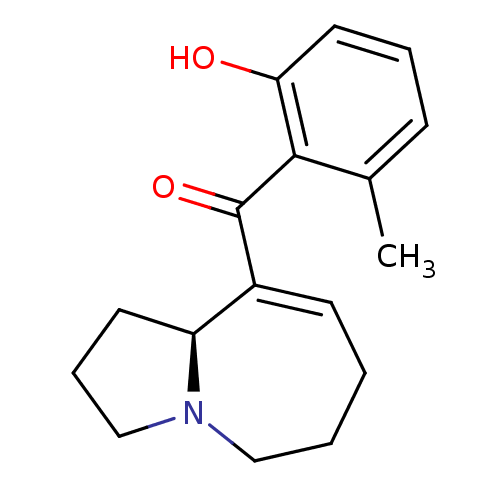 ((S,E)-(2,3,5,6,7,9a-hexahydro-1H-pyrrolo[1,2-a]aze...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50211219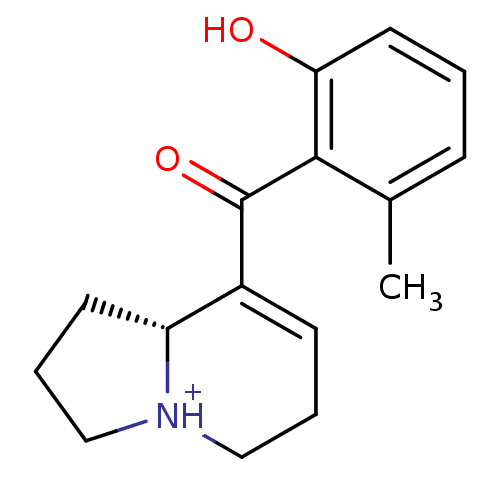 (elaeocarpenine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313167 ((S,E)-(2,3,5,6,7,9a-hexahydro-1H-pyrrolo[1,2-a]aze...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50211219 (elaeocarpenine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50211219 (elaeocarpenine) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50313164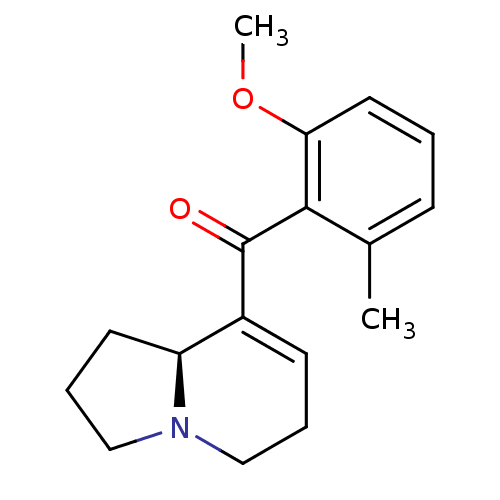 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-metho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50313163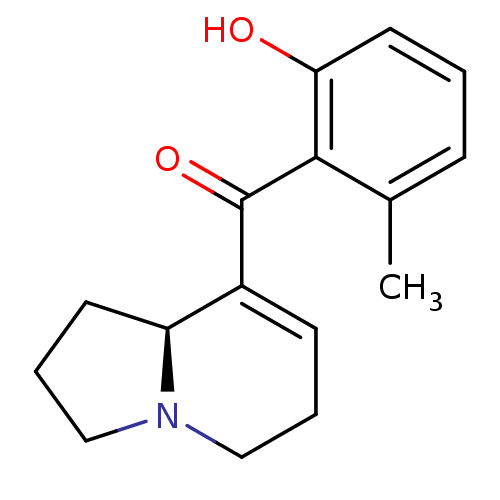 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50313166 ((S)-(2-hydroxy-6-methylphenyl)(2,3,5,7a-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313163 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313165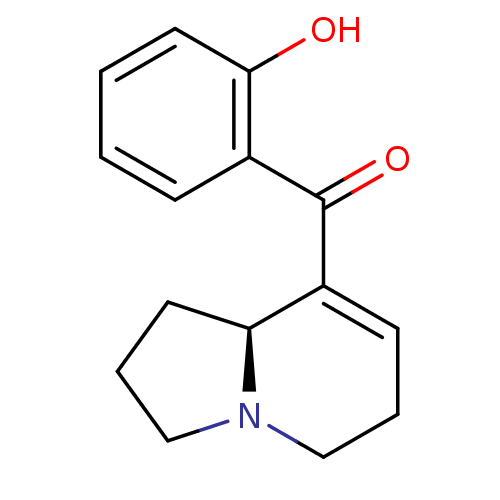 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-hydro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50313165 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from rat kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50313163 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50313167 ((S,E)-(2,3,5,6,7,9a-hexahydro-1H-pyrrolo[1,2-a]aze...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50313165 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-hydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50313166 ((S)-(2-hydroxy-6-methylphenyl)(2,3,5,7a-tetrahydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50313164 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-metho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human delta opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50313164 ((S)-(1,2,3,5,6,8a-hexahydroindolizin-8-yl)(2-metho...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 20: 1601-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.062 BindingDB Entry DOI: 10.7270/Q2K35TRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50483304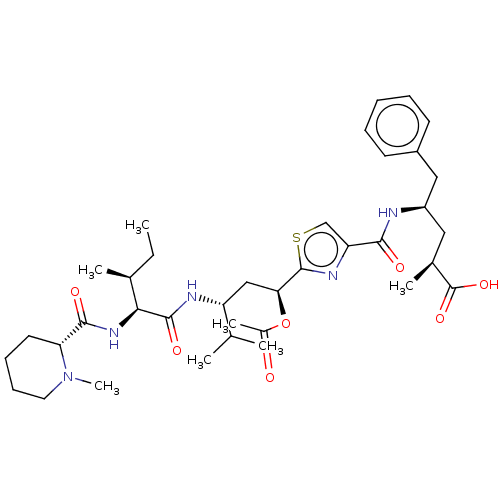 (11-Epi-Tubulysin D) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50012278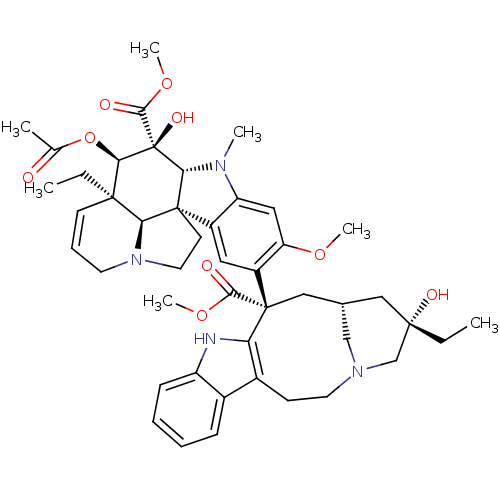 ((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50483305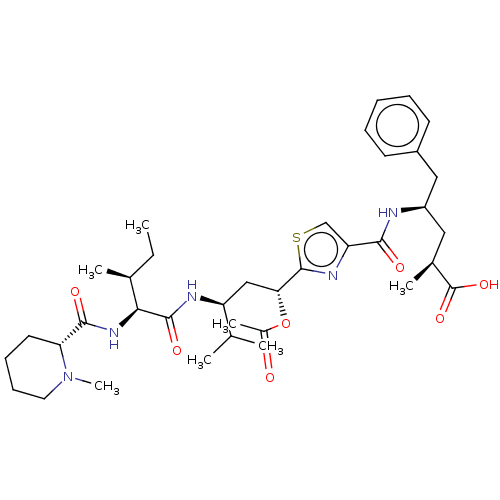 (13-Epi-Tubulysin D) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50483306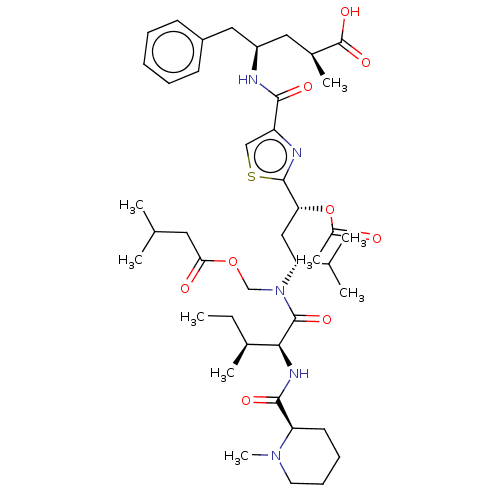 (CHEBI:80036 | Tubulysin D) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50483307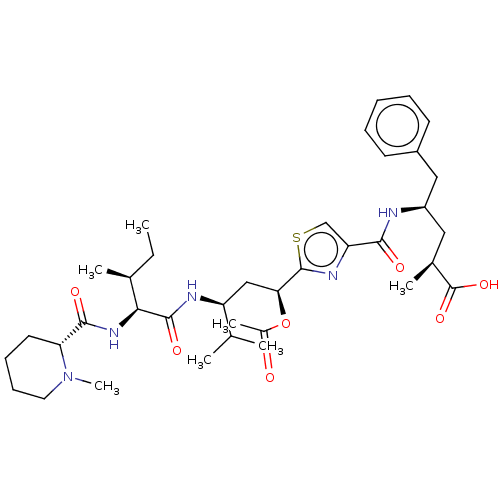 (CHEMBL1643767) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co Ltd Curated by ChEMBL | Assay Description Inhibition of porcine brain tubulin polymerization by microtubule assembly assay | Bioorg Med Chem Lett 21: 431-4 (2011) Article DOI: 10.1016/j.bmcl.2010.10.118 BindingDB Entry DOI: 10.7270/Q21J9DMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||