

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549529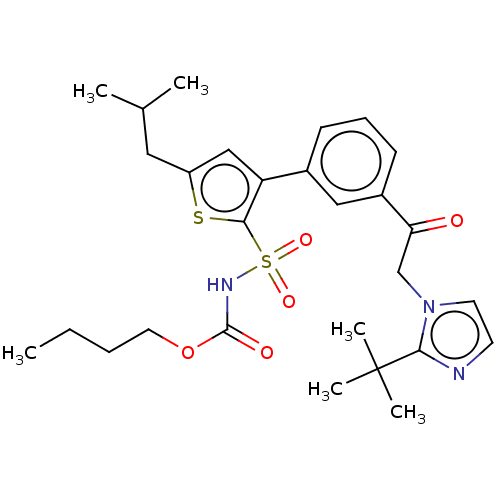 (CHEMBL4779819) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549533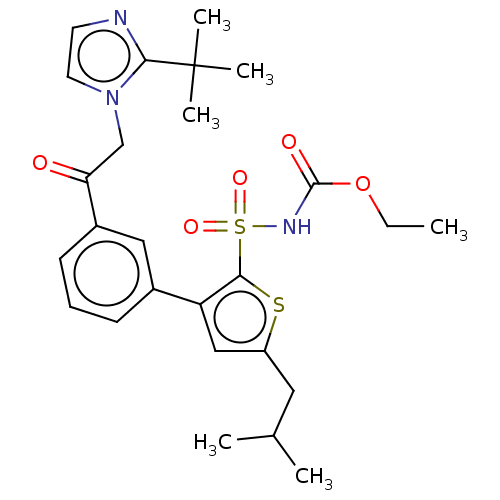 (CHEMBL4779945) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549530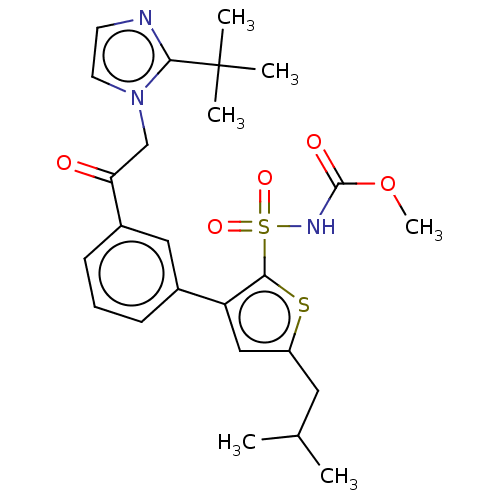 (CHEMBL4788892) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549528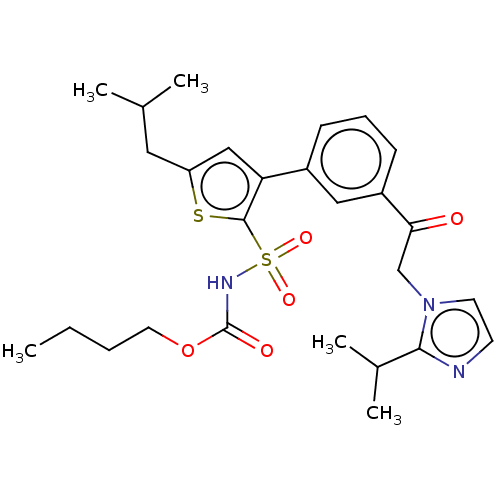 (CHEMBL4759641) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549526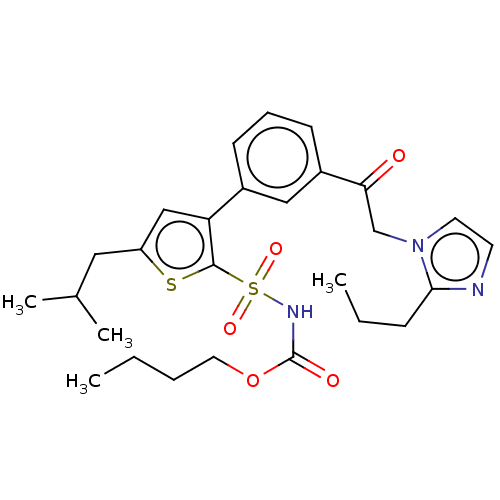 (CHEMBL4751226) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549527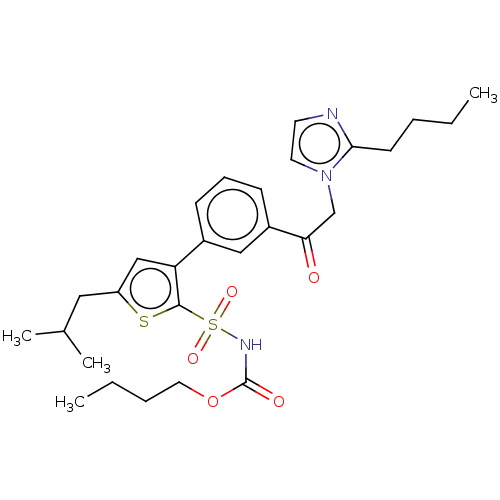 (CHEMBL4784233) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549525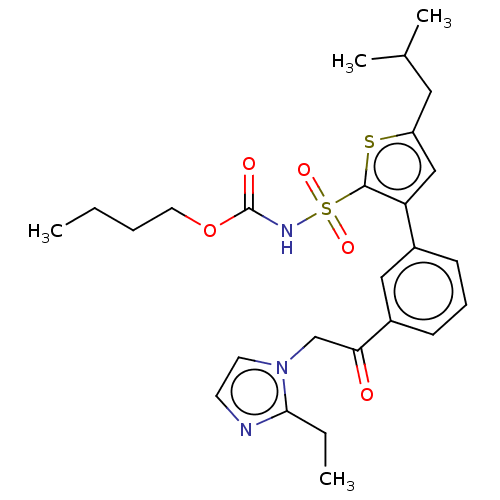 (CHEMBL2086911) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549532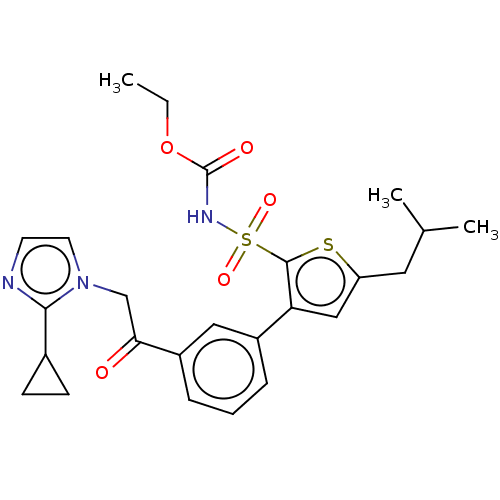 (CHEMBL4747530) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549531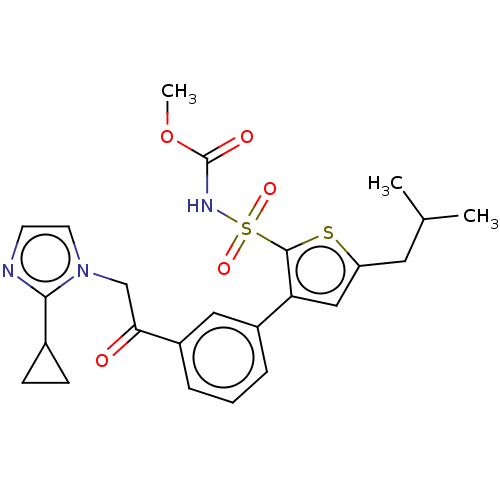 (CHEMBL4762752) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549534 (CHEMBL4797684) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030814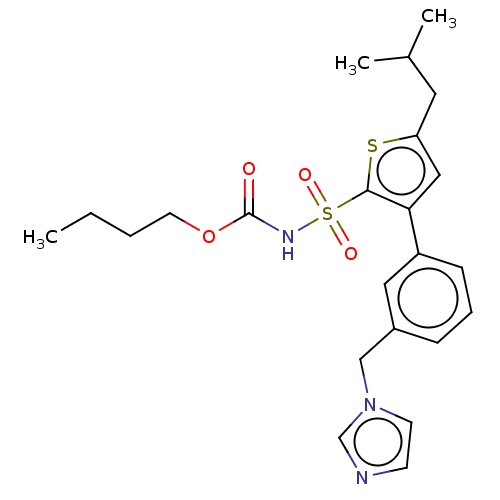 (CHEMBL2086892) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549524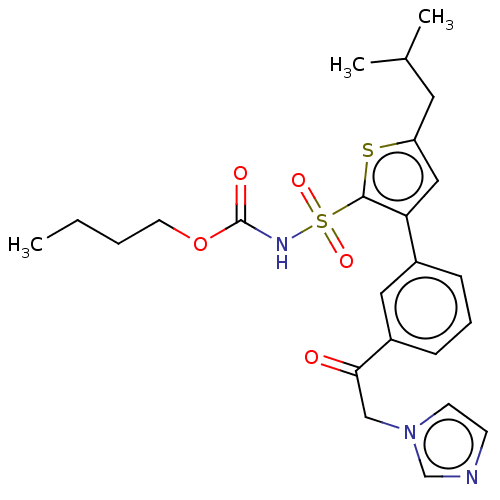 (CHEMBL2086910) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT2 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50549530 (CHEMBL4788892) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]Sar-Ile-angiotensin 2 from human AT1 receptor expressed in HEK293 cells incubated for 240 mins by radiometric scintillation ana... | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50030814 (CHEMBL2086892) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate preincubated for 3 mins followed by NADPH addition by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50030814 (CHEMBL2086892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 3 mins followed by NADPH addition by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50549530 (CHEMBL4788892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 3 mins followed by NADPH addition by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50030814 (CHEMBL2086892) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate preincubated for 3 mins followed by NADPH addition by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50549530 (CHEMBL4788892) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2D6 in human liver microsomes using bufuralol as substrate preincubated for 3 mins followed by NADPH addition by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50549530 (CHEMBL4788892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 3 mins followed by NADPH addition by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2B6 (Homo sapiens (Human)) | BDBM50549530 (CHEMBL4788892) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate preincubated for 3 mins followed by NADPH addition by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50030814 (CHEMBL2086892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 3 mins followed by NADPH addition by LC-MS/MS analysis | Citation and Details Article DOI: 10.1016/j.bmc.2020.115859 BindingDB Entry DOI: 10.7270/Q2BR8WRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||