 Found 73 hits with Last Name = 'choo' and Initial = 'ih'
Found 73 hits with Last Name = 'choo' and Initial = 'ih' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50098551

((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...)Show SMILES CC1CCN(CC[C@H]2CCCN2S(=O)(=O)c2cccc(O)c2)CC1 |r| Show InChI InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1B (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1A (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1A (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058162
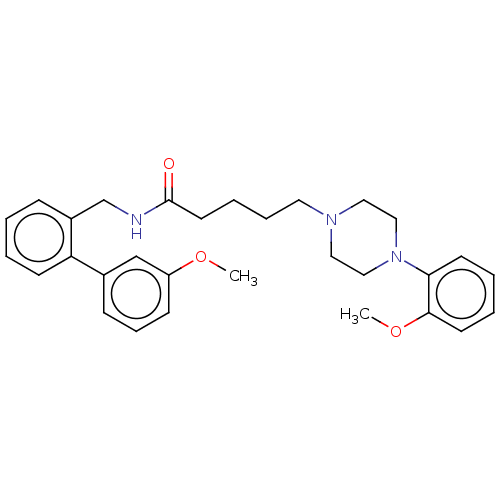
(CHEMBL3326986)Show SMILES COc1cccc(c1)-c1ccccc1CNC(=O)CCCCN1CCN(CC1)c1ccccc1OC Show InChI InChI=1S/C30H37N3O3/c1-35-26-12-9-11-24(22-26)27-13-4-3-10-25(27)23-31-30(34)16-7-8-17-32-18-20-33(21-19-32)28-14-5-6-15-29(28)36-2/h3-6,9-15,22H,7-8,16-21,23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058156
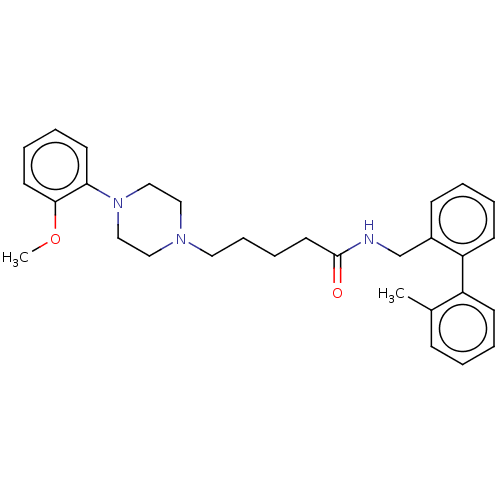
(CHEMBL3326989)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2C)CC1 Show InChI InChI=1S/C30H37N3O2/c1-24-11-3-5-13-26(24)27-14-6-4-12-25(27)23-31-30(34)17-9-10-18-32-19-21-33(22-20-32)28-15-7-8-16-29(28)35-2/h3-8,11-16H,9-10,17-23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058211

(CHEMBL3326984)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccc(Cl)cc2)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-11-5-4-10-27(28)33-20-18-32(19-21-33)17-7-6-12-29(34)31-22-24-8-2-3-9-26(24)23-13-15-25(30)16-14-23/h2-5,8-11,13-16H,6-7,12,17-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058209

(CHEMBL3326985)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2OC)CC1 Show InChI InChI=1S/C30H37N3O3/c1-35-28-15-7-5-13-26(28)25-12-4-3-11-24(25)23-31-30(34)17-9-10-18-32-19-21-33(22-20-32)27-14-6-8-16-29(27)36-2/h3-8,11-16H,9-10,17-23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058157
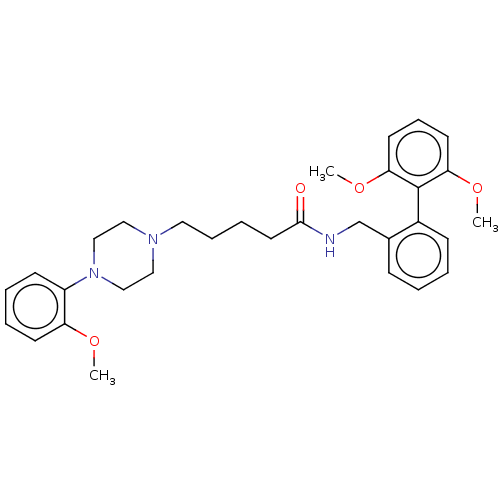
(CHEMBL3326988)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2c(OC)cccc2OC)CC1 |(26.8,-10.21,;25.47,-10.98,;25.48,-12.52,;26.82,-13.29,;26.82,-14.83,;25.49,-15.6,;24.15,-14.83,;24.16,-13.3,;22.83,-12.53,;21.49,-13.3,;20.16,-12.53,;20.16,-10.98,;18.82,-10.22,;17.49,-11,;16.16,-10.23,;14.83,-11.01,;13.49,-10.25,;13.48,-8.71,;12.16,-11.02,;10.82,-10.25,;9.49,-11.03,;9.5,-12.58,;8.16,-13.35,;6.82,-12.58,;6.83,-11.04,;8.16,-10.27,;8.15,-8.73,;6.81,-7.97,;5.47,-8.76,;5.49,-10.31,;6.8,-6.43,;8.14,-5.65,;9.48,-6.42,;9.48,-7.96,;10.81,-8.73,;12.16,-7.95,;21.49,-10.21,;22.82,-10.99,)| Show InChI InChI=1S/C31H39N3O4/c1-36-27-14-7-6-13-26(27)34-21-19-33(20-22-34)18-9-8-17-30(35)32-23-24-11-4-5-12-25(24)31-28(37-2)15-10-16-29(31)38-3/h4-7,10-16H,8-9,17-23H2,1-3H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058216

(CHEMBL3326980)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2cccc(F)c2)CC1 Show InChI InChI=1S/C29H34FN3O2/c1-35-28-14-5-4-13-27(28)33-19-17-32(18-20-33)16-7-6-15-29(34)31-22-24-9-2-3-12-26(24)23-10-8-11-25(30)21-23/h2-5,8-14,21H,6-7,15-20,22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058215

(CHEMBL3326981)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccc(F)cc2)CC1 Show InChI InChI=1S/C29H34FN3O2/c1-35-28-11-5-4-10-27(28)33-20-18-32(19-21-33)17-7-6-12-29(34)31-22-24-8-2-3-9-26(24)23-13-15-25(30)16-14-23/h2-5,8-11,13-16H,6-7,12,17-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058217

(CHEMBL3326979)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2F)CC1 Show InChI InChI=1S/C29H34FN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058219
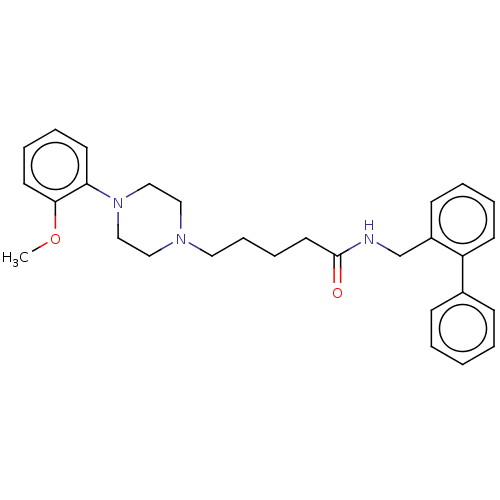
(CHEMBL3325464)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2)CC1 Show InChI InChI=1S/C29H35N3O2/c1-34-28-16-8-7-15-27(28)32-21-19-31(20-22-32)18-10-9-17-29(33)30-23-25-13-5-6-14-26(25)24-11-3-2-4-12-24/h2-8,11-16H,9-10,17-23H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058218

(CHEMBL3326978)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2)CC1 Show InChI InChI=1S/C30H37N3O2/c1-35-29-17-10-9-16-28(29)33-22-20-32(21-23-33)19-11-3-6-18-30(34)31-24-26-14-7-8-15-27(26)25-12-4-2-5-13-25/h2,4-5,7-10,12-17H,3,6,11,18-24H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058159

(CHEMBL3326987)Show SMILES COc1ccc(cc1)-c1ccccc1CNC(=O)CCCCN1CCN(CC1)c1ccccc1OC Show InChI InChI=1S/C30H37N3O3/c1-35-26-16-14-24(15-17-26)27-10-4-3-9-25(27)23-31-30(34)13-7-8-18-32-19-21-33(22-20-32)28-11-5-6-12-29(28)36-2/h3-6,9-12,14-17H,7-8,13,18-23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058070
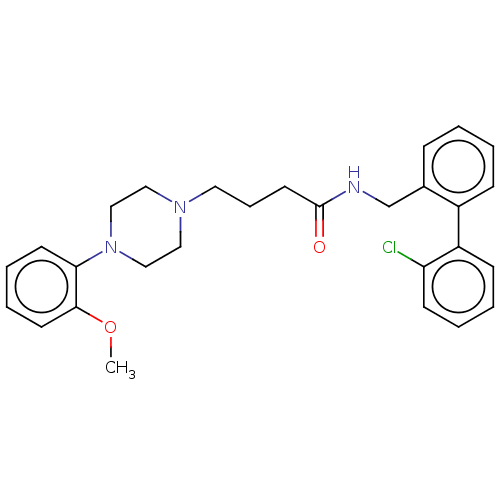
(CHEMBL3326992)Show SMILES COc1ccccc1N1CCN(CCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C28H32ClN3O2/c1-34-27-14-7-6-13-26(27)32-19-17-31(18-20-32)16-8-15-28(33)30-21-22-9-2-3-10-23(22)24-11-4-5-12-25(24)29/h2-7,9-14H,8,15-21H2,1H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058151

(CHEMBL3326990)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2cccc(C)c2)CC1 Show InChI InChI=1S/C30H37N3O2/c1-24-10-9-12-25(22-24)27-13-4-3-11-26(27)23-31-30(34)16-7-8-17-32-18-20-33(21-19-32)28-14-5-6-15-29(28)35-2/h3-6,9-15,22H,7-8,16-21,23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058282
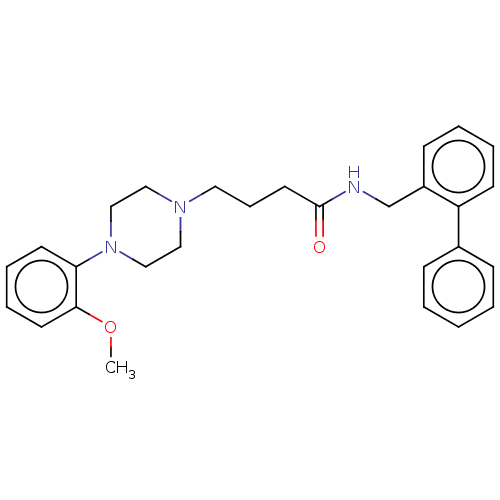
(CHEMBL3326977)Show SMILES COc1ccccc1N1CCN(CCCC(=O)NCc2ccccc2-c2ccccc2)CC1 Show InChI InChI=1S/C28H33N3O2/c1-33-27-15-8-7-14-26(27)31-20-18-30(19-21-31)17-9-16-28(32)29-22-24-12-5-6-13-25(24)23-10-3-2-4-11-23/h2-8,10-15H,9,16-22H2,1H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058071

(CHEMBL3326991)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccc(C)cc2)CC1 Show InChI InChI=1S/C30H37N3O2/c1-24-14-16-25(17-15-24)27-10-4-3-9-26(27)23-31-30(34)13-7-8-18-32-19-21-33(22-20-32)28-11-5-6-12-29(28)35-2/h3-6,9-12,14-17H,7-8,13,18-23H2,1-2H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2C (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1D (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1B (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058284
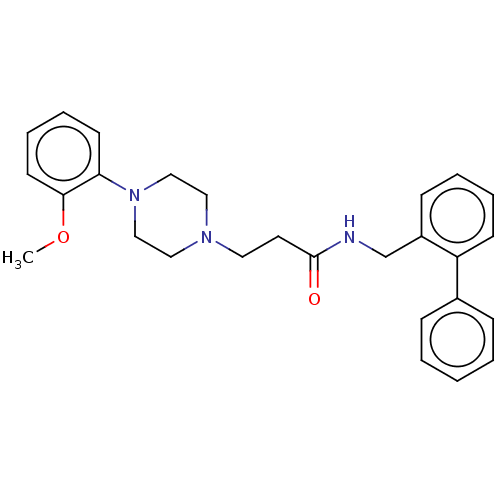
(CHEMBL3326976)Show SMILES COc1ccccc1N1CCN(CCC(=O)NCc2ccccc2-c2ccccc2)CC1 Show InChI InChI=1S/C27H31N3O2/c1-32-26-14-8-7-13-25(26)30-19-17-29(18-20-30)16-15-27(31)28-21-23-11-5-6-12-24(23)22-9-3-2-4-10-22/h2-14H,15-21H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50058213
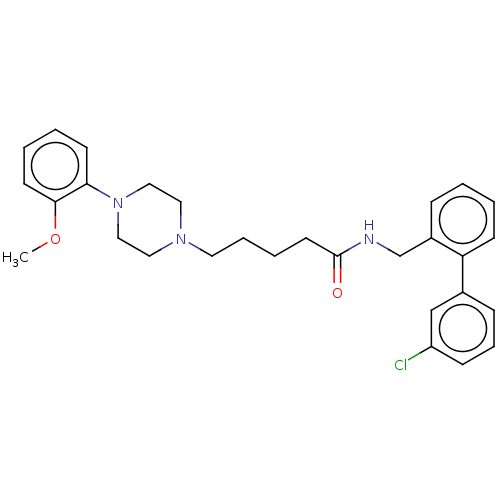
(CHEMBL3326983)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2cccc(Cl)c2)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-14-5-4-13-27(28)33-19-17-32(18-20-33)16-7-6-15-29(34)31-22-24-9-2-3-12-26(24)23-10-8-11-25(30)21-23/h2-5,8-14,21H,6-7,15-20,22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from 5-HT7R (unknown origin) expressed in CHO-K1 cells by liquid scintillation counting method |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 418 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT1D (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 478 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT2A (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 517 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT5A (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 5A
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT5A (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT6 (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT6 (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50058214

(CHEMBL3326982)Show SMILES COc1ccccc1N1CCN(CCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C29H34ClN3O2/c1-35-28-15-7-6-14-27(28)33-20-18-32(19-21-33)17-9-8-16-29(34)31-22-23-10-2-3-11-24(23)25-12-4-5-13-26(25)30/h2-7,10-15H,8-9,16-22H2,1H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT3 (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50058069

(CHEMBL3326993)Show SMILES COc1ccccc1N1CCN(CCCCCC(=O)NCc2ccccc2-c2ccccc2Cl)CC1 Show InChI InChI=1S/C30H36ClN3O2/c1-36-29-16-9-8-15-28(29)34-21-19-33(20-22-34)18-10-2-3-17-30(35)32-23-24-11-4-5-12-25(24)26-13-6-7-14-27(26)31/h4-9,11-16H,2-3,10,17-23H2,1H3,(H,32,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Binding affinity to 5-HT3 (unknown origin) |
Bioorg Med Chem 22: 4587-96 (2014)
Article DOI: 10.1016/j.bmc.2014.07.026
BindingDB Entry DOI: 10.7270/Q22N53ZG |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay |
Bioorg Med Chem 21: 5480-7 (2013)
Article DOI: 10.1016/j.bmc.2013.05.066
BindingDB Entry DOI: 10.7270/Q2QN69PN |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50345949
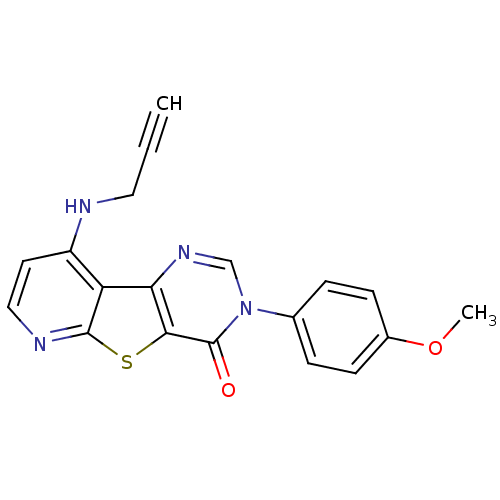
(3-(4-Methoxy-phenyl)-9-prop-2-ynylamino-3H-pyrido[...)Show SMILES COc1ccc(cc1)-n1cnc2c(sc3nccc(NCC#C)c23)c1=O Show InChI InChI=1S/C19H14N4O2S/c1-3-9-20-14-8-10-21-18-15(14)16-17(26-18)19(24)23(11-22-16)12-4-6-13(25-2)7-5-12/h1,4-8,10-11H,9H2,2H3,(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50493222
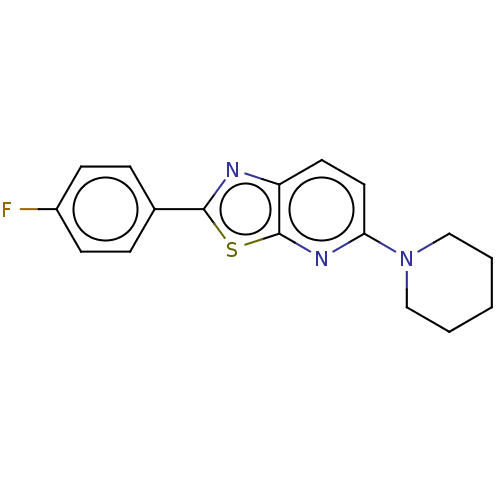
(CHEMBL2418211)Show InChI InChI=1S/C17H16FN3S/c18-13-6-4-12(5-7-13)16-19-14-8-9-15(20-17(14)22-16)21-10-2-1-3-11-21/h4-9H,1-3,10-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay |
Bioorg Med Chem 21: 5480-7 (2013)
Article DOI: 10.1016/j.bmc.2013.05.066
BindingDB Entry DOI: 10.7270/Q2QN69PN |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058042
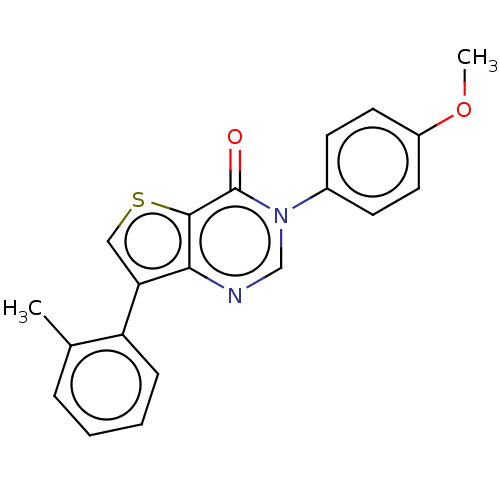
(CHEMBL3330823)Show InChI InChI=1S/C20H16N2O2S/c1-13-5-3-4-6-16(13)17-11-25-19-18(17)21-12-22(20(19)23)14-7-9-15(24-2)10-8-14/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058048

(CHEMBL3330811)Show InChI InChI=1S/C19H14N2O2S/c1-23-15-9-7-14(8-10-15)21-12-20-17-16(11-24-18(17)19(21)22)13-5-3-2-4-6-13/h2-12H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058044

(CHEMBL3330817)Show InChI InChI=1S/C19H13FN2O2S/c1-24-13-8-6-12(7-9-13)22-11-21-17-15(10-25-18(17)19(22)23)14-4-2-3-5-16(14)20/h2-11H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058056

(CHEMBL3330801)Show InChI InChI=1S/C18H11FN2OS/c19-13-7-4-8-14(9-13)21-11-20-16-15(10-23-17(16)18(21)22)12-5-2-1-3-6-12/h1-11H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058055
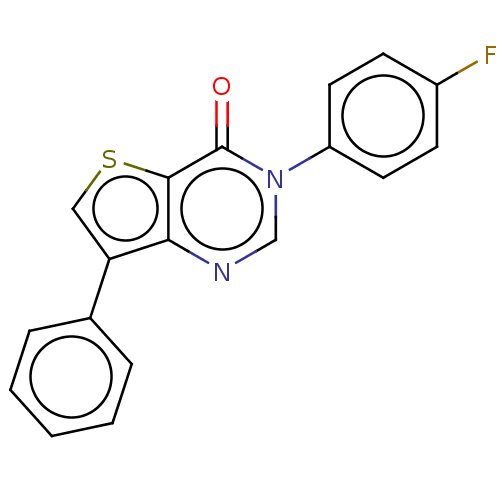
(CHEMBL3330802)Show InChI InChI=1S/C18H11FN2OS/c19-13-6-8-14(9-7-13)21-11-20-16-15(10-23-17(16)18(21)22)12-4-2-1-3-5-12/h1-11H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058047

(CHEMBL3330812 | US9260448, 29)Show InChI InChI=1S/C20H14N2OS/c1-2-14-7-6-10-16(11-14)22-13-21-18-17(12-24-19(18)20(22)23)15-8-4-3-5-9-15/h2-13H,1H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058049
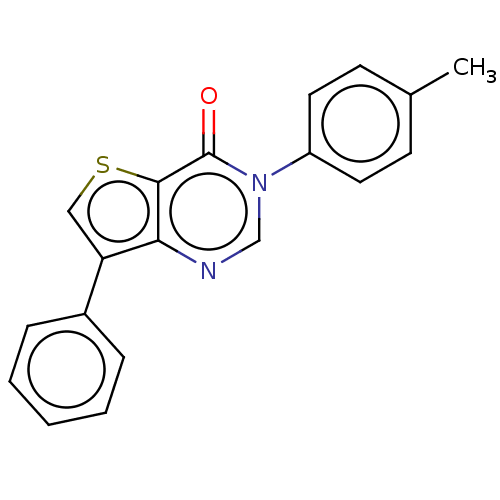
(CHEMBL3330808 | US9260448, 18)Show InChI InChI=1S/C19H14N2OS/c1-13-7-9-15(10-8-13)21-12-20-17-16(11-23-18(17)19(21)22)14-5-3-2-4-6-14/h2-12H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058045
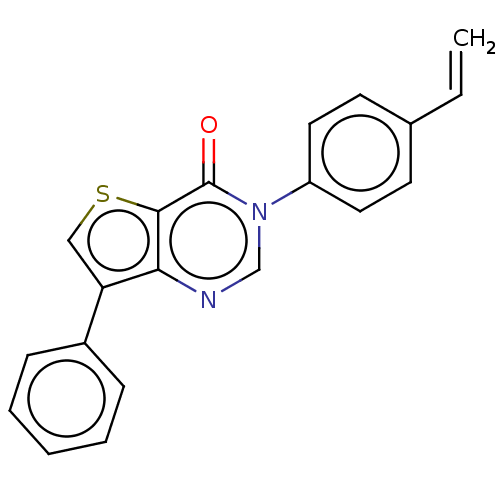
(CHEMBL3330813)Show InChI InChI=1S/C20H14N2OS/c1-2-14-8-10-16(11-9-14)22-13-21-18-17(12-24-19(18)20(22)23)15-6-4-3-5-7-15/h2-13H,1H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058059

(CHEMBL3330800)Show InChI InChI=1S/C18H12N2OS/c21-18-17-16(15(11-22-17)13-7-3-1-4-8-13)19-12-20(18)14-9-5-2-6-10-14/h1-12H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(Homo sapiens (Human)) | BDBM50058052
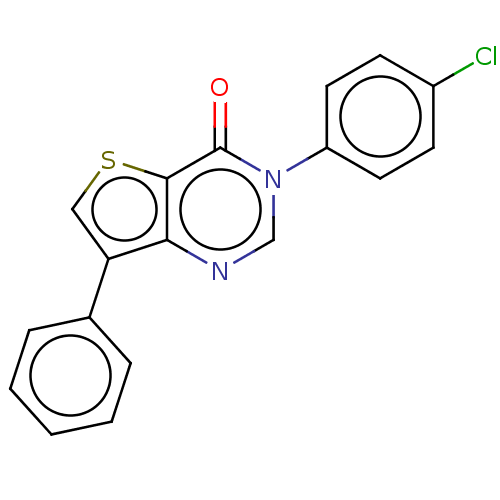
(CHEMBL3330805 | US9260448, 7)Show InChI InChI=1S/C18H11ClN2OS/c19-13-6-8-14(9-7-13)21-11-20-16-15(10-23-17(16)18(21)22)12-4-2-1-3-5-12/h1-11H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University
Curated by ChEMBL
| Assay Description
Antagonist activity at mGluR1 (unknown origin) expressed in Chem-3 cells assessed as inhibition of glutamate-induced increased intracellular calcium ... |
Eur J Med Chem 85: 629-37 (2014)
Article DOI: 10.1016/j.ejmech.2014.08.027
BindingDB Entry DOI: 10.7270/Q2B56MDJ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50493221
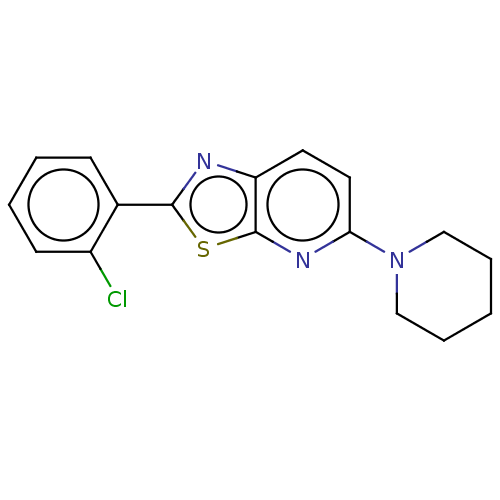
(CHEMBL2418212)Show InChI InChI=1S/C17H16ClN3S/c18-13-7-3-2-6-12(13)16-19-14-8-9-15(20-17(14)22-16)21-10-4-1-5-11-21/h2-3,6-9H,1,4-5,10-11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-B using benzylamine hydrochloride as substrate assessed as H2O2 synthesis after 1 hr by fluorescence assay |
Bioorg Med Chem 21: 5480-7 (2013)
Article DOI: 10.1016/j.bmc.2013.05.066
BindingDB Entry DOI: 10.7270/Q2QN69PN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data