

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxin B (Peptoclostridium difficile) | BDBM50454459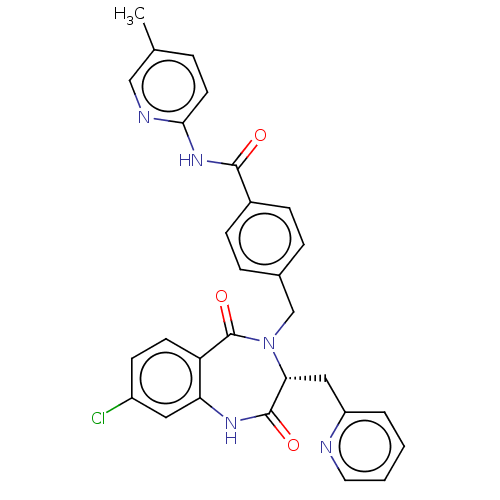 (CHEMBL4215036) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454459 (CHEMBL4215036) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507130 (CHEMBL4452983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507108 (CHEMBL4529863) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454498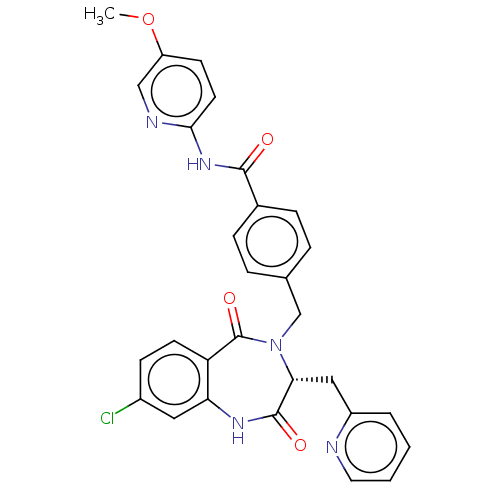 (CHEMBL4215657) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454497 (CHEMBL4214079) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454500 (CHEMBL4212258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507128 (CHEMBL4451752) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507134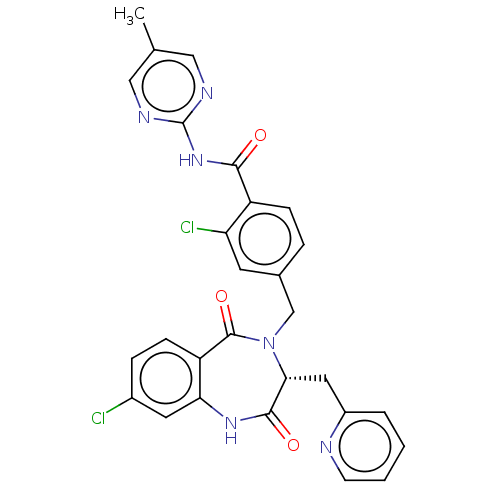 (CHEMBL4444965) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507115 (CHEMBL4520413) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507107 (CHEMBL4473557) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507135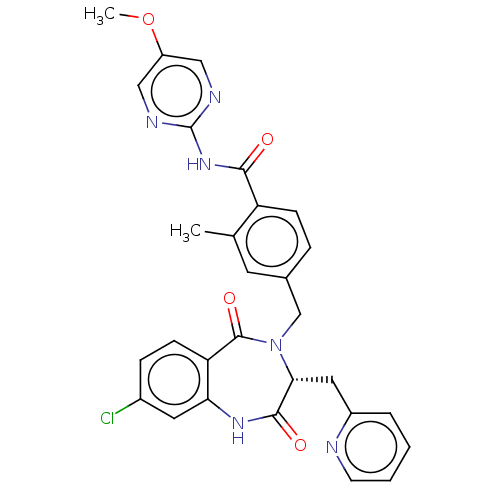 (CHEMBL4470638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507120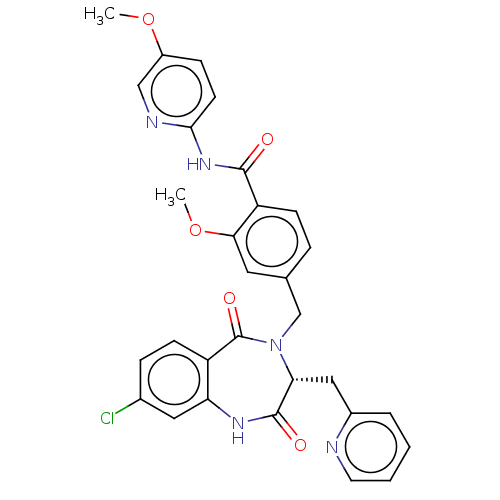 (CHEMBL4473443) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507105 (CHEMBL4557223) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507127 (CHEMBL4476228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507133 (CHEMBL4461315) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507136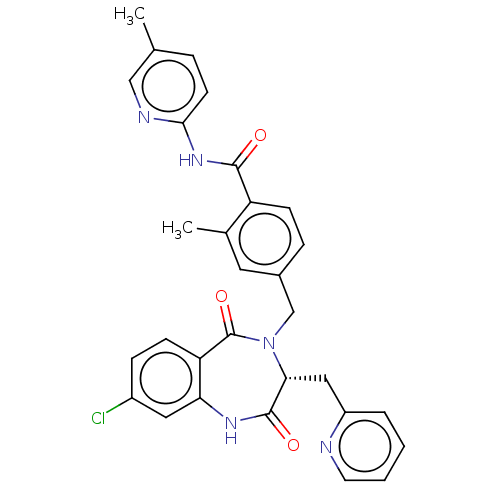 (CHEMBL4454294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454497 (CHEMBL4214079) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507126 (CHEMBL4483028) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507114 (CHEMBL4553525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507113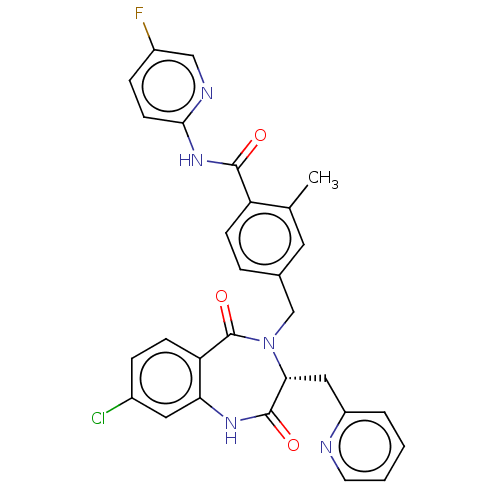 (CHEMBL4436890) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454486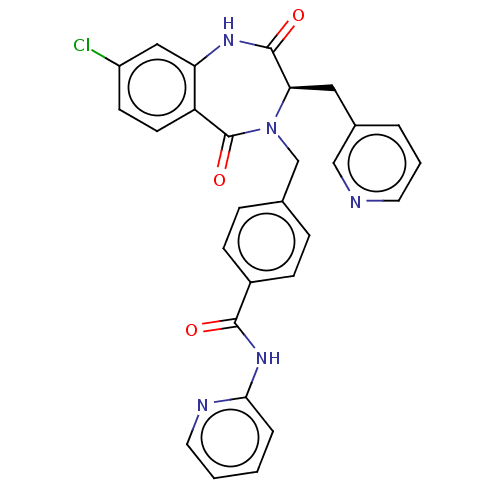 (CHEMBL4203819) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507116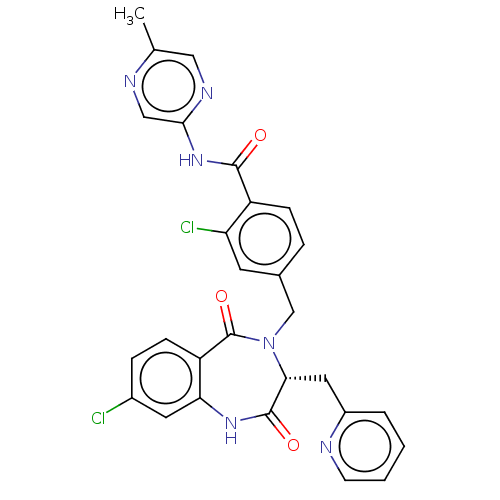 (CHEMBL4452876) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507119 (CHEMBL4441033) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507106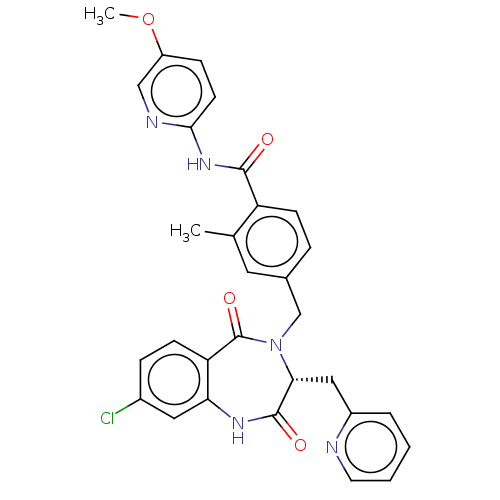 (CHEMBL4472977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507129 (CHEMBL4456814) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507112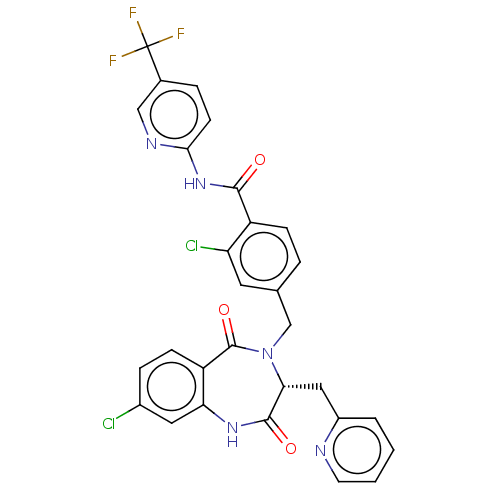 (CHEMBL4547929) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454504 (CHEMBL4210388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50413094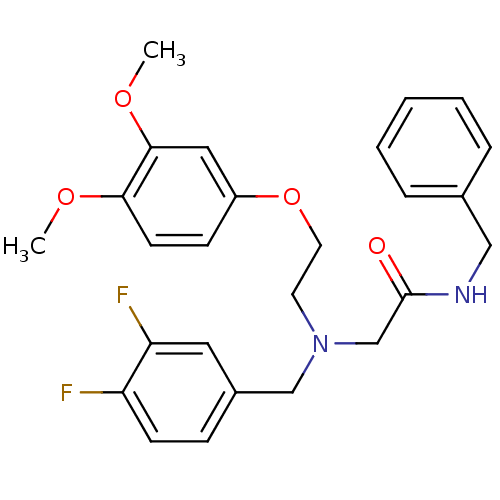 (CHEMBL486116) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK293 cells assessed as inhibition of human orexin-A-stimulated calcium release by FLIPR assa... | Bioorg Med Chem Lett 18: 5420-3 (2008) Article DOI: 10.1016/j.bmcl.2008.09.038 BindingDB Entry DOI: 10.7270/Q27082N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50413093 (CHEMBL528990) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK293 cells assessed as inhibition of human orexin-A-stimulated calcium release by FLIPR assa... | Bioorg Med Chem Lett 18: 5420-3 (2008) Article DOI: 10.1016/j.bmcl.2008.09.038 BindingDB Entry DOI: 10.7270/Q27082N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50175173 (4-(4-((2,4-dichlorophenethyl)carbamoyl)-2-(3-chlor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Inhibition of CXCL10(IP-10)-stimulated calcium release in HEK293 cells expressing recombinant human CXCR3 and chimeric G protein Gqi5 | Bioorg Med Chem Lett 16: 200-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.020 BindingDB Entry DOI: 10.7270/Q29Z94GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507118 (CHEMBL4456808) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507117 (CHEMBL4591470) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507105 (CHEMBL4557223) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50413092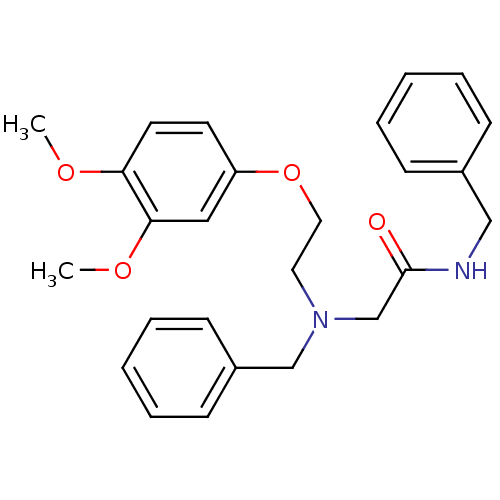 (CHEMBL519258) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK293 cells assessed as inhibition of human orexin-A-stimulated calcium release by FLIPR assa... | Bioorg Med Chem Lett 18: 5420-3 (2008) Article DOI: 10.1016/j.bmcl.2008.09.038 BindingDB Entry DOI: 10.7270/Q27082N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507135 (CHEMBL4470638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507109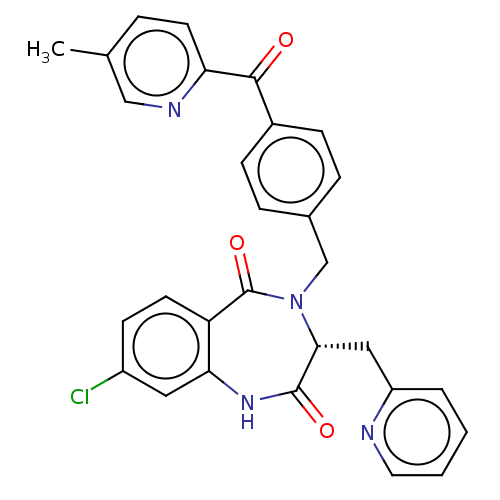 (CHEMBL4530206) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507121 (CHEMBL4589634) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454503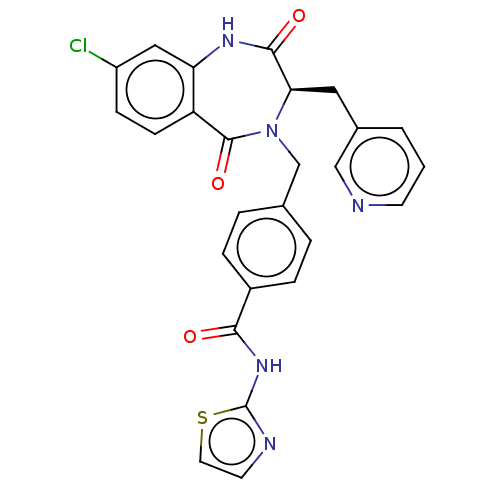 (CHEMBL4211420) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454498 (CHEMBL4215657) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507110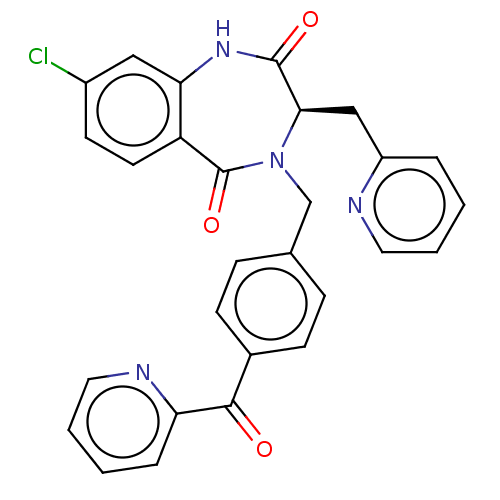 (CHEMBL4545776) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B glucosyltransferase domain assessed as reduction in UDP-glucose hydro... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50454502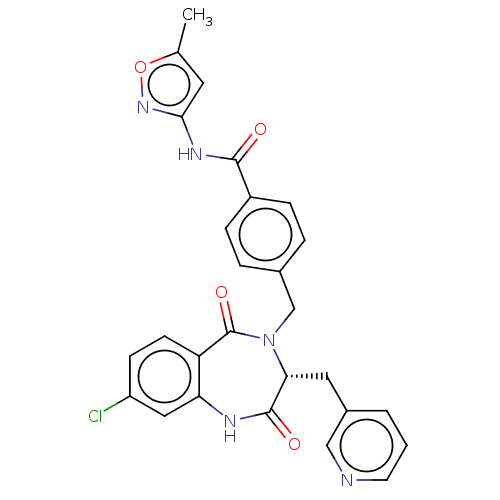 (CHEMBL4209041) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Genesis Biotechnology Group Curated by ChEMBL | Assay Description Inhibition of C-terminal 6-His tagged recombinant Clostridium difficile toxin B catalytic fragment (Met1 to Leu543 residues) assessed as reduction in... | Bioorg Med Chem Lett 28: 756-761 (2018) Article DOI: 10.1016/j.bmcl.2018.01.005 BindingDB Entry DOI: 10.7270/Q2QR50RG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50175193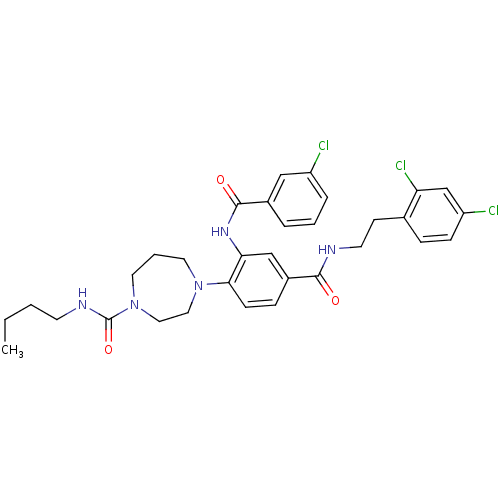 (4-(4-((2,4-dichlorophenethyl)carbamoyl)-2-(3-chlor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Inhibition of CXCL11-stimulated calcium release in HEK293 cells expressing recombinant human CXCR3 and chimeric G protein Gqi5 | Bioorg Med Chem Lett 16: 200-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.020 BindingDB Entry DOI: 10.7270/Q29Z94GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50413100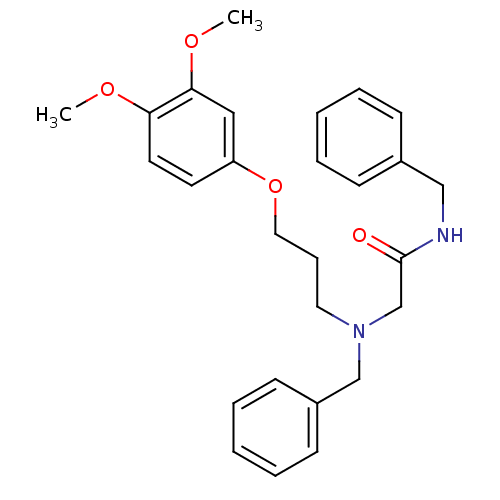 (CHEMBL485915) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia, Inc. Curated by ChEMBL | Assay Description Antagonist activity at human OX2 receptor expressed in HEK293 cells assessed as inhibition of human orexin-A-stimulated calcium release by FLIPR assa... | Bioorg Med Chem Lett 18: 5420-3 (2008) Article DOI: 10.1016/j.bmcl.2008.09.038 BindingDB Entry DOI: 10.7270/Q27082N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507114 (CHEMBL4553525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxin B (Peptoclostridium difficile) | BDBM50507115 (CHEMBL4520413) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Venenum Biodesign Curated by ChEMBL | Assay Description Inhibition of Clostridium difficile toxin B transfected in CHO cells assessed as reduction in caspase 3/7 activation pre-incubated for 1 hr before Tc... | Bioorg Med Chem Lett 28: 3601-3605 (2018) Article DOI: 10.1016/j.bmcl.2018.10.047 BindingDB Entry DOI: 10.7270/Q2KP85FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50175183 (4-(4-((2,4-dichlorophenethyl)carbamoyl)-2-(3-chlor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Inhibition of CXCL11-stimulated calcium release in HEK293 cells expressing recombinant human CXCR3 and chimeric G protein Gqi5 | Bioorg Med Chem Lett 16: 200-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.020 BindingDB Entry DOI: 10.7270/Q29Z94GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50175168 (4-(4-((2,4-dichlorophenethyl)carbamoyl)-2-(3-chlor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Inhibition of CXCL11-stimulated calcium release in HEK293 cells expressing recombinant human CXCR3 and chimeric G protein Gqi5 | Bioorg Med Chem Lett 16: 200-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.020 BindingDB Entry DOI: 10.7270/Q29Z94GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50175176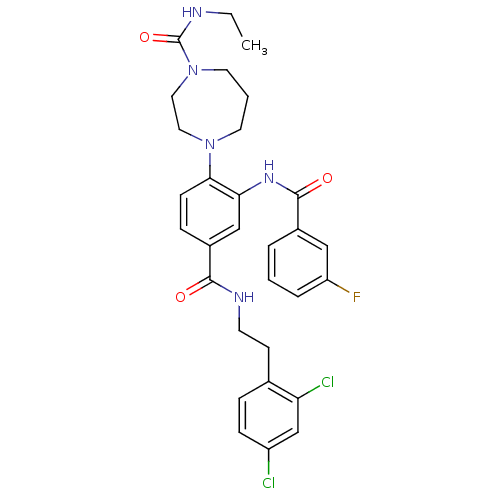 (4-(4-((2,4-dichlorophenethyl)carbamoyl)-2-(3-fluor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Inhibition of CXCL11-stimulated calcium release in HEK293 cells expressing recombinant human CXCR3 and chimeric G protein Gqi5 | Bioorg Med Chem Lett 16: 200-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.020 BindingDB Entry DOI: 10.7270/Q29Z94GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50175173 (4-(4-((2,4-dichlorophenethyl)carbamoyl)-2-(3-chlor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc. Curated by ChEMBL | Assay Description Inhibition of CXCL11-stimulated calcium release in HEK293 cells expressing recombinant human CXCR3 and chimeric G protein Gqi5 | Bioorg Med Chem Lett 16: 200-3 (2005) Article DOI: 10.1016/j.bmcl.2005.09.020 BindingDB Entry DOI: 10.7270/Q29Z94GD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |